
Health & Medicine
Tracking avian influenza to safeguard Australia

Avian flu is continuing to spread throughout the world, infecting some mammals as it goes – but what’s the risk to Australia?
Published 20 March 2023
Avian flu is continuing to spread throughout the world, killing wild birds and poultry en masse across the northern hemisphere, Africa and South America.
Not only is this strain of bird flu wreaking havoc on wild birds and poultry, it is responsible for numerous cases in mammals, including humans.

But what is the threat to Australia?
Bird flu is a form of avian influenza that’s highly pathogenic for poultry and many wild birds.
Since 2021, we have seen the unfolding of an enormous panzootic of a particular strain of this highly pathogenic avian influenza, known as HPAI clade 2.3.4.4b H5N1.

Health & Medicine
Tracking avian influenza to safeguard Australia
There are current outbreaks on all continents except, for now, the Antarctic and Australia.
These outbreaks have been responsible for the deaths of more than half a billion poultry as of 28 Feb 2023, either due to the virus itself or in attempts to stop the virus spread through culling.
The number of wild bird deaths is unknown, but the scale is potentially in the millions across a great diversity of birds. So far, deaths have been recorded in some 303 different bird species.
For example, a recent outbreak in Peru reported more than 50,000 dead wild birds across the country’s protected areas. These outbreaks are of substantial conservation concern for wild birds, with potential population effects and, in some cases, a risk of extinction.
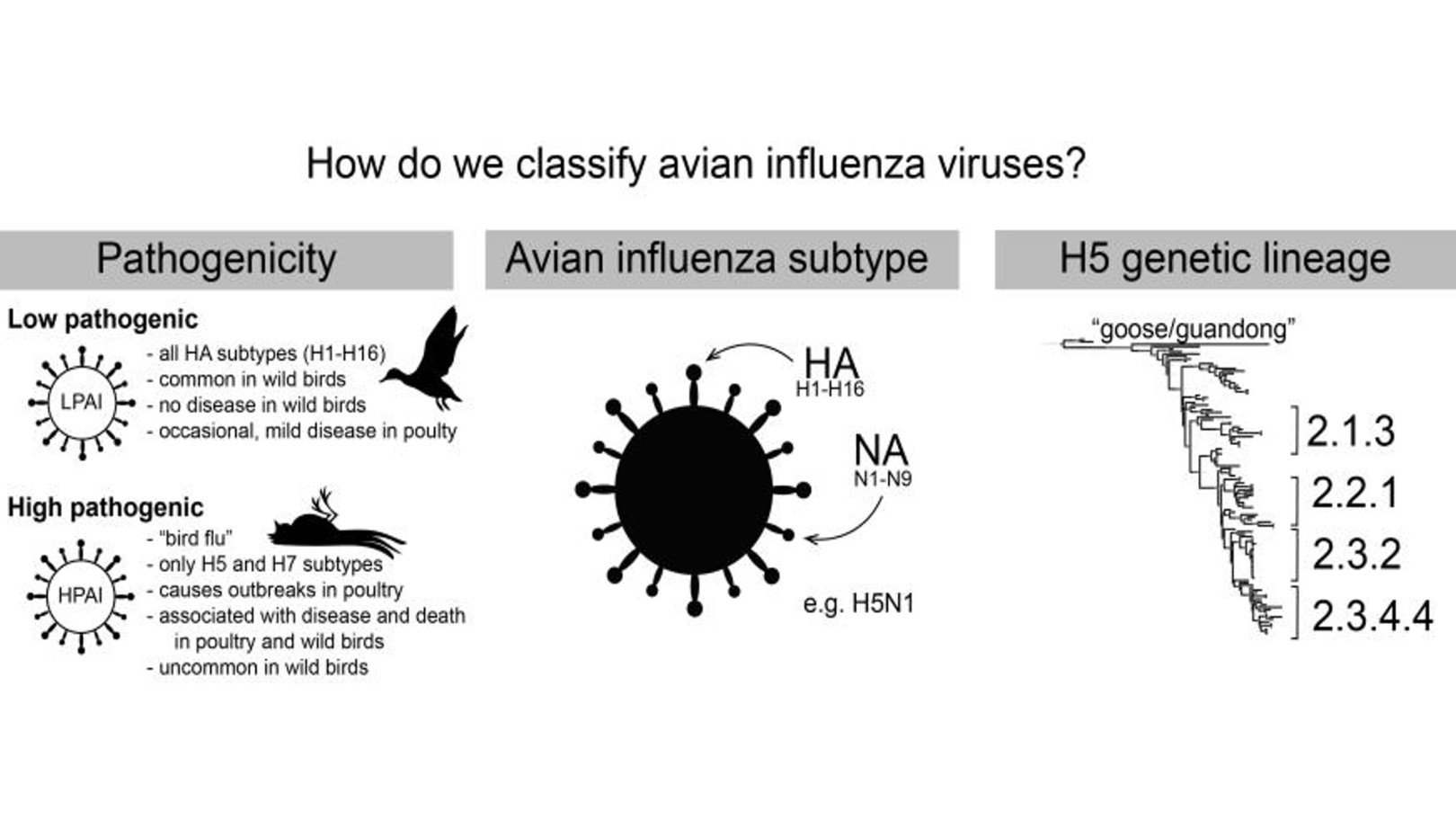
Of note, is that while HPAI viruses usually cause very high mortality in chickens and other poultry, with varying mortality in wild birds, there is an enormous diversity of avian influenza strains that cause no disease and circulate naturally in wild birds. These are called low pathogenic avian influenza (or LPAI) viruses.
In addition to the avian outbreaks, numerous cases have been detected in mammals. These include outbreaks in a large Spanish mink farm, in sea lions in South America and a variety of human cases, most recently, a child in Cambodia.

Health & Medicine
How our ‘avian athletes’ could spread influenza
These outbreaks have caused global concern about any potential mammal-to-mammal transmission.
Currently, this virus is still a bird adapted virus – meaning there are no confirmed cases of mammal-to-mammal transmission. The vast majority of cases reported in mammals have been in predators and scavengers that have been infected by the dead or dying birds that they’ve eaten.
So, perhaps it’s not surprising to see an increase in the number of mainly carnivorous mammalian cases given the scale of wild-bird outbreaks.
From experimental studies, we have a good idea which mutations are required for mammal-to-mammal transmission.
One of these mutations is currently widely detected in mammalian cases and occurs at lower mammalian body temperatures instead of the hotter avian body temperatures.

The other mutations of concern, which could make avian influenza viruses more transmissible in mammals, have not yet been found in the wild, but continued genomic surveillance and mutation mapping is critical to track the ongoing evolution of this virus.
To date, all human cases have been in people exposed to sick or dead birds (mainly poultry) infected with HPAI and there have been no confirmed instances of human-to-human transmission.
HPAI H5Nx has caused around 900 human infections since 2003, with an overall case fatality rate of approximately 60 per cent.

Health & Medicine
Viruses on the wing
Given the large number of avian outbreaks, it’s reassuring that the number of human cases is very few. Even some of the human cases that have been initially recorded as infections with the 2.3.4.4b H5N1 viruses may have been due to carriage of the virus in the nose rather than a true infection.
This is often referred to as “environmental contamination”, resulting in a positive test for H5N1 virus in nasal swabs but the person shows no symptoms of a true infection, as has been suggested in two recent cases in Spain.
The World Health Organization (WHO) states that the risk for humans remains low, but is elevated for people occupationally exposed to infected birds. However, the WHO also indicates that the zoonotic risk is elevated.
Importantly, there are a number of therapeutics, like specific influenza antiviral drugs, which work well against all avian influenza viruses including H5N1 if taken early enough.

The WHO also evaluates avian influenza viruses at its bi-annual vaccine strain meeting in preparation for a potential pandemic and prepares seed viruses that could be used to produce vaccines if required.
Avian influenza is clearly a OneHealth disease – that is it affects wildlife, domestic animals and humans. So a multi-pronged OneHealth response is critical; we cannot wait until signs of human-to-human transmission occur.

Health & Medicine
Penguin viruses in the frozen continent
Strong biosecurity is key to preventing viral incursion into poultry production and subsequent spill-over into wild birds – there’s certainly capacity for continued improvements.
More recently, vaccination has been considered as an additional tool to control spread.
Some countries, like Mexico, Vietnam and China have been vaccinating flocks for some time to try to control HPAI.
The US, UK, European Union (EU) and Australia do not vaccinate, however EU legislation has recently been changed to accommodate vaccination, but strict conditions must be met to ensure that vaccination efforts are effective and do not further drive virus evolution or cause “silent spread” (diminishes disease but does not stop infection).
These measures include making sure the vaccine is well matched to the circulating HPAI and that vaccination actually prevents infection with HPAI.

While not a silver bullet, vaccination has the potential to alleviate the burden of HPAI H5Nx on poultry when done appropriately and in combination with other exisiting approaches.
This will likely help prevent human spillover infections and would also hopefully reduce the impact on wild birds by lowering the overall environmental viral load.
Currently, Australia remains free from HPAI H5N1.
While avian influenza outbreaks have occurred here previously, they have always been caused by domestic strains that have evolved to become highly pathogenic rather than an incursion from globally circulating HPAI strains.

Health & Medicine
Don’t blame the pangolin (or any other animal) for COVID-19
However, just because it hasn’t happened before, doesn’t mean this particular virus (that has become increasingly capable to also infect wild birds) will not arrive in Australia.
Millions of wild birds migrate between Asia and Australia each year, arriving from their northern hemisphere breeding grounds between September and November.
The main reason why this virus has not yet reached Australia is likely because there are no migratory duck species, which are the main movers of influenza viruses, migrating between East Asia and Australia.
However, we do know that shorebirds and seabirds are hosts for influenza, so if HPAI H5N1 were to arrive in Australia, it would most likely arrive with them.
In response to this risk, our team has performed enhanced surveillance on wild migratory birds as they arrived in Australia between September and December last year.
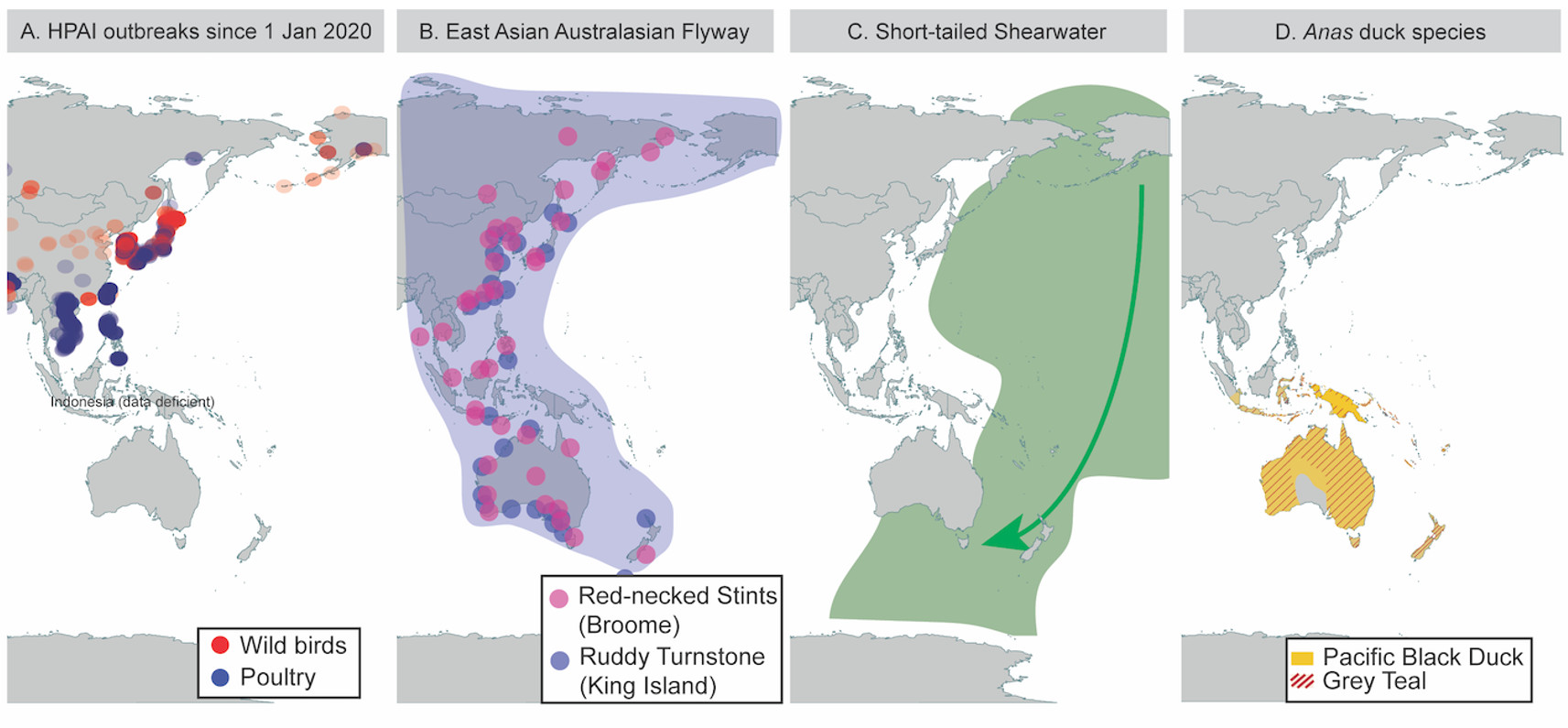
Thankfully all birds tested negative and there have been no indications of outbreaks in Australia since.
We will again enter a risk period of viral introduction when the migratory birds return in the second half of this year, and our teams will again be on the front lines to provide state and federal agencies rapid information to help them respond to any outbreaks – if they occur.
HPAI looks like it will continue to circulate for many years to come, so we need to maintain our long-term vigilance in Australia and work with others to try and eradicate or control this global threat.
Banner: Getty Images