Health & Medicine
Putting cells through their paces
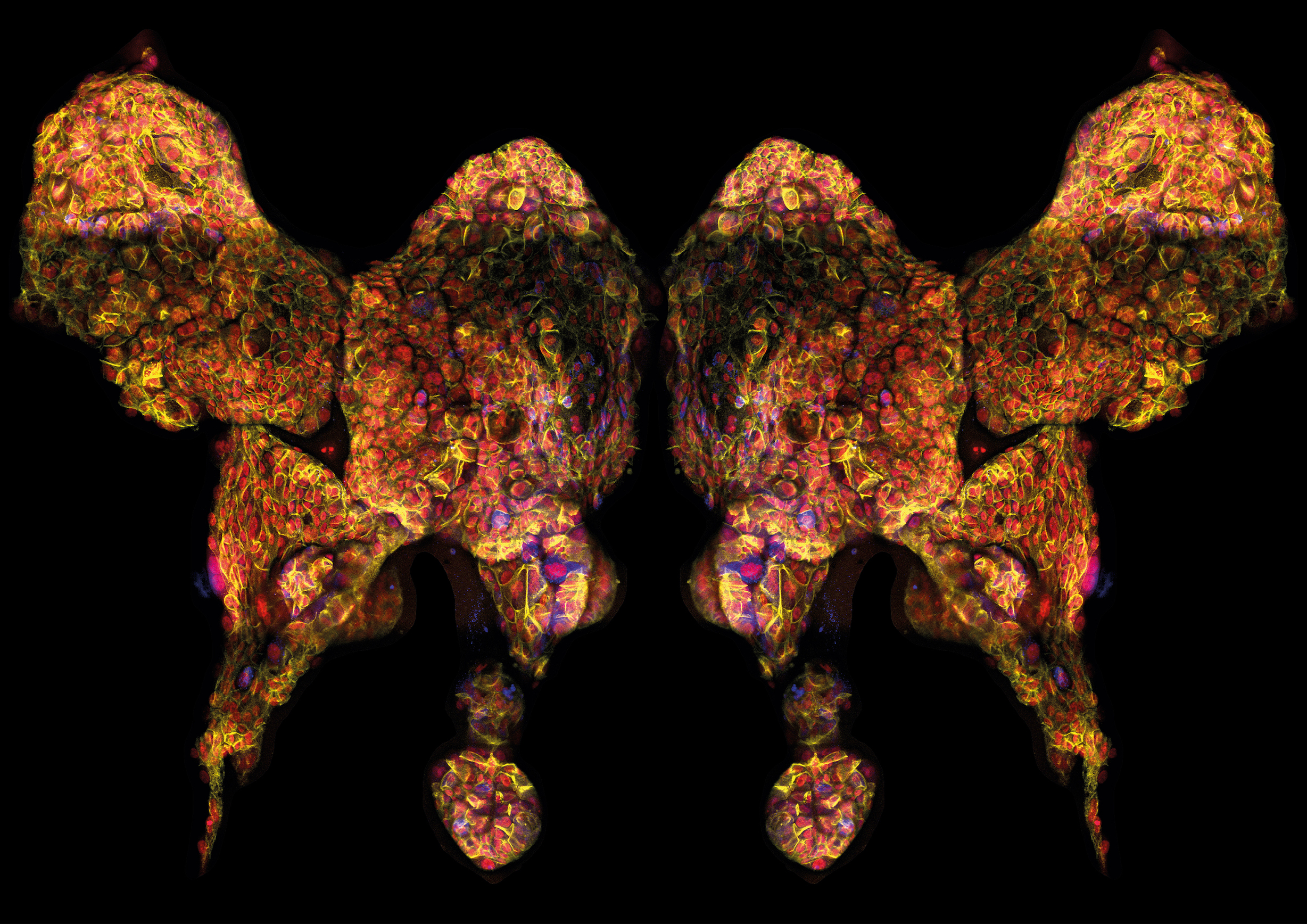
A stem cell that makes mistakes in repairing DNA has been uncovered as the likely culprit behind a major lung cancer
Published 28 January 2017
For four years straight medical researcher Clare Weeden would go on alert whenever lung surgery was underway anywhere across Melbourne. No matter the time, she would have to be ready in her lab to receive samples of fresh tissue as part of a project to isolate and research the stem cells that repair our lungs as they constantly breathe in contaminants from air pollution to cigarette smoke.
She didn’t know it at the time, but she was hot on the trail of the lung’s basal stem cells that now appear to be the likely culprits that trigger a major lung cancer closely tied to smoking – squamous cell carcinoma. It is the second most common form of lung cancer.
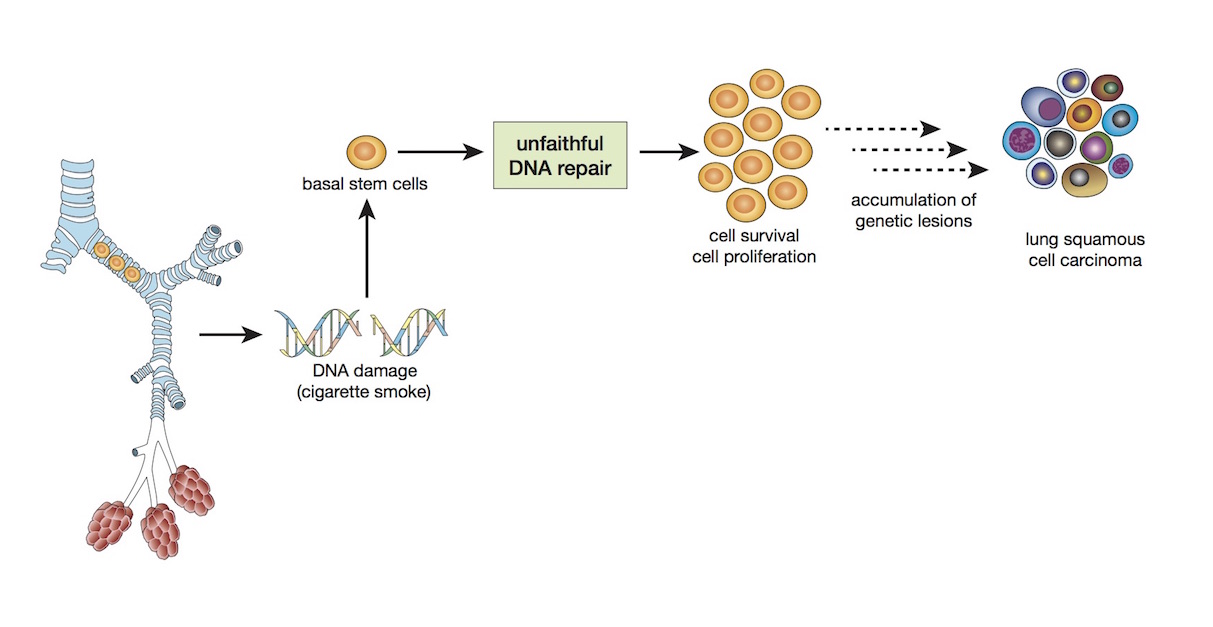
Basal stems cells are very quick at repairing DNA damage caused by inhaled chemicals such as those from cigarette smoke, but they are prone to making mistakes. It means that the more repair work they have to do, the greater the chance of a cancer-causing mutation.
“What we have found is a genetic fingerprint in squamous cell carcinoma that has been left from basal stem cells in the lung whose repair work has gone awry and led to the cancer,” says Ms Weeden, from the Walter and Eliza Hall Institute of Medical Research and a PhD candidate at the University of Melbourne.
“It isn’t definitive but the evidence is that lung basal stem cells are the likely cells of origin.”
The unmasking of basal stem cells, published in the US-based Public Library of Science: Biology, is the culmination of years of painstaking laboratory work and data-crunching that has now provided a crucial new target for developing drugs that may be able to turn off the progress of the cancer.
Ms Weeden was sometimes up until to 3am at the Institute isolating and processing cells from the freshly operated-on lung tissue, especially when there was a flurry of samples in one day. It is a complex process that took up to six hours for each of the eventual 140 samples.
To extract and isolate the cells she first had to mince the tissue into tiny pieces with scalpels before adding enzymes and other media. She then put the material through micro strainers and a centrifuge, after which the cells could then be stained with antibodies and passed through a laser cell-sorting machine. She would then have to get the stem cells to grow into colonies to prove they were indeed stem cells and that her isolation process was working.

Health & Medicine
Putting cells through their paces
But one day she came across a sample that she could barely get to grow at all.
Intrigued, she contacted the Victorian Cancer Biobank for basic information on the donor. It was likely that the donor was a smoker or ex-smoker since most people having lung surgery have a history of smoking. But this patient had never smoked. Sensing a possible link she went back to the Biobank to get information on all the previous tissue donors, and over that weekend plotted out a chart.
The correlation was stark. Samples from those that had never smoked had low basal cell growth, and the more heavily a patient had smoked, the higher the growth rate.
“It completely grabbed my curiosity,” she says. “I remember on Monday morning going straight into my supervisor’s office (Dr Marie-Liesse Asselin-Labat) and putting the chart down in front of her. We both realised we were onto something significant. The question was what?”
By using the same process that Weeden had developed to accurately isolate lung stem cells, she and Dr Asselin-Libat set to examining how the basal stem cells worked.
They discovered that basal stem cells were very efficient at repairing damaged DNA but that the process the cells use, called non-homologous repair, is prone to making errors that can lead to cancer-causing mutations. In non-homologous repair the break in a damaged DNA chain is simply closed over rather than copied. They also found evidence of the accumulation of mutations in the basal stem cells of the smokers.
“While we need more experimentation, this gave us a model of what may be happening,” says Weeden. “Our lungs are constantly being exposed to what we inhale. When we breathe in something like cigarette smoke that causes lung damage, these basal cells receive a signal to grow and repair the damage.
But they have to first repair their own DNA damage and the process they use is very quick. The advantage is that it helps the cells to survive, but the disadvantage is that they are prone to making errors that can lead to cancer.”
To test that model they turned to Institute bioinfomaticians Professor Gordon Smyth and Dr Yunshun (Andy) Chen who used statistics and computer science to extract a genetic “signature” for lung basal stem cells. They then compared that signature with the genetics of various lung cancers.
They discovered that this same signature was highly correlated with lung squamous cell carcinoma, the second most common form of lung cancer and the most closely linked to smoking – some 96 per cent of people with lung squamous cell carcinoma are either smokers or ex-smokers. It was clear evidence that basal stem cells are the likely culprits in how the cancer is triggered.
By identifying a cell of origin Weeden says we now have a drug target to aim at that has the potential to stop the progress of the cancer. Previous Institute research in 2009 lead by Professor Jane Visvader and Professor Geoff Lindeman had similarly identified a likely cell of origin for inherited breast cancer, and last year that same team identified an existing drug, denosumab, that in laboratory models could switch off the problematic cell growth and curtail the cancer. Clinical trials are now underway.
“In the breast cancer research they similarly used correlations to identify a cell of origin like we have and now further work has solidified that,” says Weeden.
Does this mean that at some point in the future smokers could breathe easier by taking a drug that could stop the cancer being triggered? No. Weeden points out that if someone took such a drug and continued to smoke the damage could be even worse than the cancer.
“Basal stem cells have a job to do in the lung, they repair any damage. If a person was treated with a drug that turned off basal cells and continued to smoke, I would imagine that other lung problems may develop due to the inability of the stem cell to repair the lung airways from cigarette smoke-induced damage,” says Weeden. She points out that smoking also causes other lung cancers that don’t arise from basal stem cells.
She says the biggest beneficiaries of any such drug could be ex-smokers. “This is particularly relevant as lung squamous cell carcinoma can occur in ex-smokers who have quit perhaps 20 or 30 years ago.
“But the best way to reduce the risk of lung cancer is to simply quit smoking because no matter how long you’ve smoked for, the risk of lung cancer is reduced when you quit.”
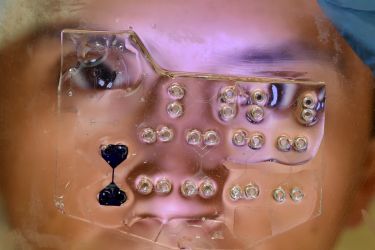
Banner Image: Colonies of lung cells grown in the laboratory from a single lung stem cell from a cancer patient Picture: Clare Weeden, Walter and Eliza Hall Institute.