Health & Medicine
New targets for epilepsy treatment
A novel method for understanding the function of the neural pathways could help reveal new information about behaviour and diseases of the brain
Published 21 September 2020
The brain is an extraordinarily complex organ and neuroscientists are constantly striving to gain new understanding of what specific regions do.
We know, for example, the hippocampus is involved in the consolidation of memory, but within the hippocampus the levels of complexity keep expanding.

Every brain region has a stunningly diverse array of nerve cells, called neurons, that fulfil different roles and contribute to the overall function of that particular part of the brain.

Health & Medicine
New targets for epilepsy treatment
Unravelling this complexity at a cellular level is a major goal of neuroscience and may unlock questions like what causes a cell group to become active during a particular behaviour or disease.
This huge undertaking requires novel technological tools to yield new insights.
There are numerous approaches to understanding brain function, like functional magnetic resonance imaging which allows us to observe which brain regions are activated during a specific task – like when we learn something new…..perhaps about chemogenetics.
Within experimental neuroscience, key advances in understanding the brain in greater detail, that is at a cellular level, have come from new techniques known as optogenetics and chemogenetics.
As their name suggests, both have a ‘genetic’ basis where researchers introduce the genes needed to produce novel molecules into a specific group of cells. This can be achieved using viruses that can’t replicate, but are used to transport new genes into living cells of animal models.
With this technique, researchers can express molecules that are activated by light, in a fashion similar to the cells in our eyes, that then alter the activity of the nerve cell when it is exposed to light of a particular wavelength.

Health & Medicine
Buffering against brain change in schizophrenia
This is the ‘opto’ part and different molecules enable excitation (turning on), or inhibition (turning off) of neuron activity.
Excitation provides information about what a particular cell type can do under general conditions, while inhibition provides information about what a cell is actually doing under any circumstance, for example, when we recall a memory.
Chemogenetics provides similar control of the cell; but, as the ‘chemo’ suggests, rather than using light to alter the cell’s activity, it uses a chemical that only affects the cell expressing the novel molecule, and not others.
Using these approaches, neuroscientists have made substantial gains in understanding the function of different nerve cell groups.
But, how do we take the next step and ask “what is causing the specific cell to be active under a particular condition, be that during a behaviour or possibly in a disease state?
Going some way towards answering this, our latest work published in Cell Reports, describes a new form of chemogenetics that enables neuroscientists to address these questions.

Our form of chemogenetics involves the ‘switching on’ or expression of small insect proteins in the brains of mammals. We have used the allatostatin system which produces a peptide, or small protein, involved in insect development.
Health & Medicine
Will COVID-19 change healthcare for stroke patients?
When the allatostatin receptor is exposed to allatostatin, neural cell activity is inhibited. Expressing the allatostatin receptor in mammalian brains has no effect on brain function because no nerve cells make allatostatin, and there’s no other chemical made that acts on the receptor, so it is inert.
Similarly, delivering the chemical allatostatin to mammals without the introduced receptor does not do anything because there are no natural receptors for it – it is just degraded.
Neuroscientists have used viruses to express allatostatin receptors in specific nerve cells. The receptor sits inactive until the neuroscientist injects the allatostatin, at which time the cells expressing the receptor, and only those cells, are inhibited and stop functioning.
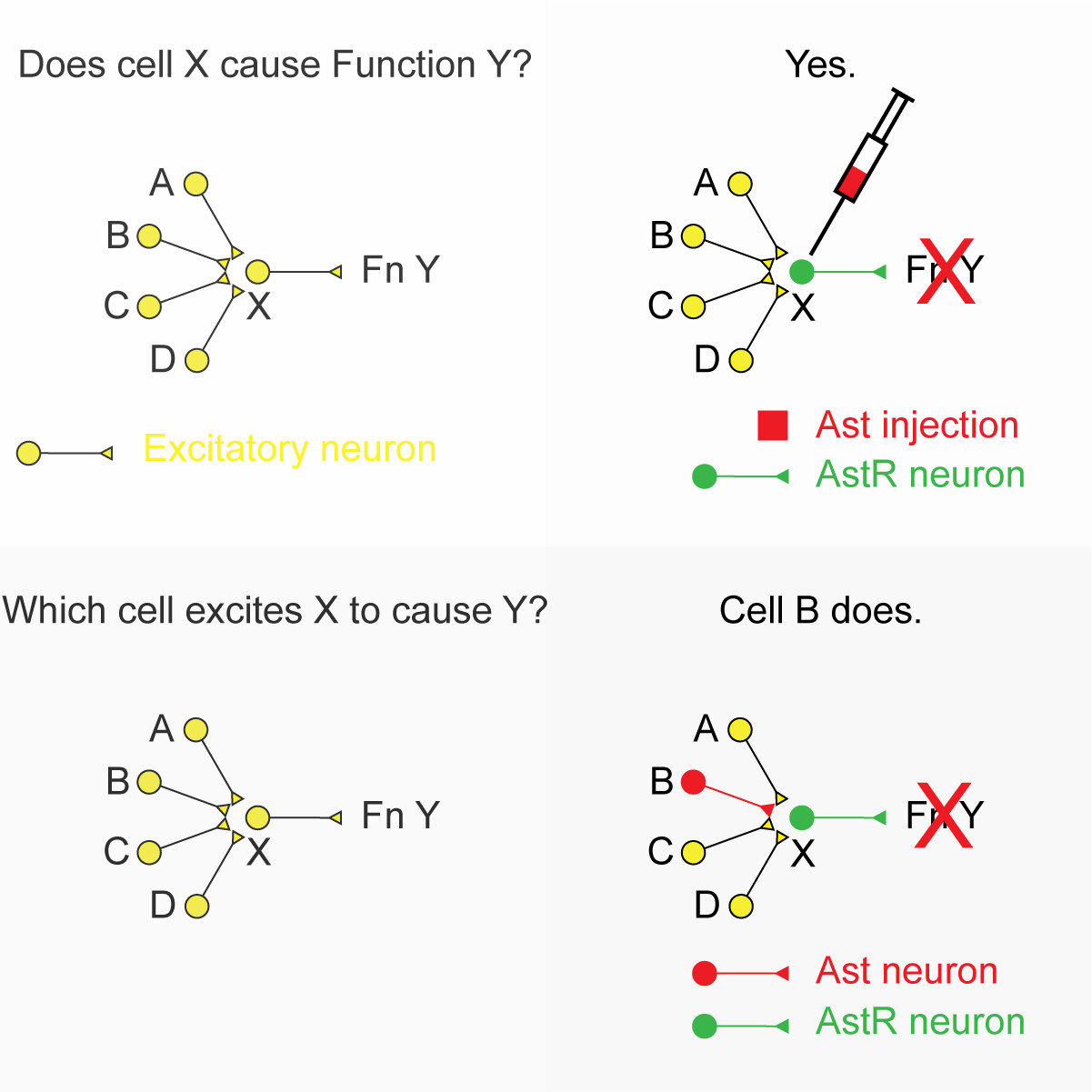
Whatever role those cells were responsible for ceases and we can say “under these conditions, cell group X is responsible for function Y” (see Figure 1).
In this technique, the allatostatin is delivered by injection and diffuses throughout the brain tissue until it reaches the receptor. As such, it doesn’t provide any information about the nerve pathways that are responsible for cell group X becoming active.
We reasoned that if we could cause nerve cells to make and release allatostatin as a neurotransmitter we could address this issue.
We used a range of methods to make a virus that enables this.
In this scenario, where cell group X is causing function Y, we might know that cell group X is excited by an input from cell groups A, B, C, D – but we don’t know which of these cells groups provides the drive to cause function Y (Figure 1).
Health & Medicine
Investigating the brain’s insulation
By expressing the allatostatin receptor in cell group X and the allatostatin in cell group B, we can directly address this question and gain new knowledge about the organisation of brain pathways responsible for specific functions.
We used this new chemogenetic approach to show that ongoing sensory input from the gastrointestinal system to a specific group of nerve cells in the brainstem is responsible for the maintenance of normal body weight.
When this pathway is inhibited, animal models show sustained decreases in body weight that were maintained for the length of the experiment.
Using viruses to cause expression of insect peptides and proteins in nerve cells of living mammals might seem like science fiction, but it is feasible. These kind of developments are providing amazing new insights into brain function and the complexity of this incredible organ.
While our research is not directed at human application or therapy, viral approaches to cause expression of proteins, for example for the production of COVID-19 vaccines, or to treat rare genetic conditions, are becoming part of human therapy and are shown to be safe.
In the future, a single injection of a safe, innocuous virus could be used to treat conditions like obesity, for life.
Banner: Getty Images