Health & Medicine
A smarter way to deliver drugs
Many of the most common cancers need a specific molecule to grow and spread, and cutting off the supply is showing promise as a new way to treat the disease
Published 27 November 2019
Just like us, cancer has things it can’t live without.
They’re not things like coffee or chocolate, but some cancers are ‘addicted’ to particular molecules. And the hope is that if it can be denied these compounds, the cancer will die.
Professor Elizabeth Vincan set out to understand how to curb cancer’s addictions more than 20 years ago.
Her research group was among a number to realise that some cancer cells always ‘switched on’ specific genes that function in an ancient form of cell-to-cell communication.
If researchers could find out what these genes did, and block them, it could provide a new way to treat cancer.

Health & Medicine
A smarter way to deliver drugs
“One of the traits of a malignant cancer is that it can grow and move, known as metastasis. This process emulates how an embryo develops when cells replicate and move to become different parts of the body – like the heart, skin and nerve cells,” Professor Vincan says.
“We know that some of the processes that are essential in embryo development are the same ones that go wrong in cancer growth. How we make an organ is a similar process to how cancer develops, but in cancer we grow too many cells in the wrong place.”
One set of genes that researchers have found to be over-active in many types of cancer is the Frizzled family of genes – the family is named for studies in fruit flies that found the genes helped determine the shape of the insect’s frizzled wing hairs.
Frizzled genes produce so-called FZD receptors that sit inside the membranes of cells. When the receptor binds to a substance that stimulates cell growth, called Wnt, they fit like a key in a lock and trigger changes in cell behaviour.
These Frizzled genes are of interest because during embryo development they help determine cell shape and movement in a process that can be hijacked by cancer.
Scientists like Professor Vincan describe these types of cancers as ‘Wnt addicted’ because they can’t grow without it. These ‘addicted’ cancers include melanoma, lung, ovarian, prostate, breast and gastric (stomach lining) cancer.
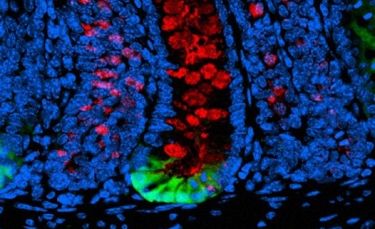
Health & Medicine
Targeting dormant cancer cells
Professor Vincan explains that these receptor-Wnt combinations are part of the instructions that tell cells where to go, what to do and what to be.
In their latest research, Professor Vincan and her collaborators focussed on a specific FZD receptor – FZD7 – in gastric cancer, investigating how it could be blocked to prevent cancer growth.
“Gastric cancer is one of the top four most common cancers globally, often due to it being diagnosed at a later stage,” says Professor Vincan.
“Patients with gastric cancer have a very poor five-year survival rate with few approved therapies that target it, so it became a major focus for our group.”
Previous research had tended to overlook the potential role of the receptor-Wnt in gastric cancer growth. Instead much of the focus has been on a defect in a gene called APC that normally produces a tumour suppressor to stop cells from growing and dividing too fast or in an abnormal way.
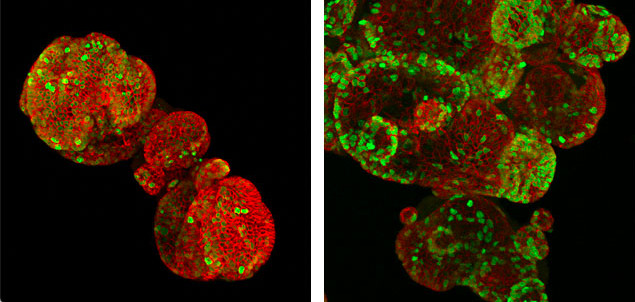
A person with this defect can’t suppress the overgrowth of cells that leads to the formation of abnormal growths, or polyps, in the gastric lining or colon, which can then become cancerous.
A defective APC means that the activity of the growth promoter Wnt becomes uncontrolled, allowing cancer to multiply.
So, it was assumed that the receptor-Wnt combination wasn’t involved in the initiation of cancer growth, instead its growth was blamed on the defective APC. But Professor Vincan and her team have found that this assumption wasn’t the whole story.

Health & Medicine
Gene genies: Meet the researchers mapping our DNA to combat cancer
“Our team noticed that the Wnt and receptor gene signals were still very active in tumours with the APC mutation, so we wondered if they still had a part to play in cancer growth,” Professor Vincan says.
“In our latest study, we developed gastric cancer organoids, tiny replicas of tissue grown in the lab, that allowed us to study these cancers in 3D.”
The team focussed on a particular FZD receptor called FZD7 because it is known to be overexpressed (which means to make too many copies of the protein) in gastric cancers.
To understand how much FZD7 was involved in stimulating cancer growth, they used the drug Vantictumab – an antibody that neutralises FZD7 – blocking the lock-and-key combination where it binds to the Wnt growth factor.
They also physically removed the receptor FZD7 completely by deleting the gene that codes for it in a mouse model.

“We were thrilled to find that both of these chemical and physical methods stopped the initiation and growth of gastric tumours in the organoid model,” says Professor Vincan.
She adds that these results reveal for the first time that FZD7 performs additional stimulation of cancer growth, irrespective of normal or defective APC.

Health & Medicine
Wiping cancer from our hard drives
The next piece of the puzzle is how to target the receptor FZD7 for future therapies.
Vantictumab is currently in clinical trials for advanced pancreatic, lung and breast cancer, but not for gastric cancer.
“These results show that patients with gastric cancer may also benefit from the current therapeutic approach using Vantictumab,” Professor Vincan says.
“It offers a new opportunity to improve the targeted management of cancers.”
Readers should note that the three phases of clinical trials can take anywhere from 10 to 15 years, but the time it takes for a drug to be tested and approved varies greatly.
The research team on the gastric cancer work included former lab members Dr Dustin Flanagan, Dr Toby Phesse and collaborators Professor Hans Clevers (Hubrecht Institute, Netherlands) and Professor Nick Barker (Institute of Medical Biology, Singapore).
Banner: Wellcome Trust