Health & Medicine
Putting cancer cells to sleep

Brain cancer kills more children in Australia than any other disease, but genetically engineered killer T-cells could be a game changer
Published 26 May 2019
“The brain is a very high stakes environment to be working in when it comes to the immune system, because the side effects of potential new treatments, like inflammation, can be devastating when in the brain.”
Immunologist Dr Misty Jenkins is talking about the difficulty of finding a cure for one of the deadliest of cancers – brain cancer.

More children die from brain cancer in Australia than from any other disease. Some 154 children aged one to 14 years old died from the disease in the period 2011 to 2015, ahead of 122 deaths caused by leukaemia.
And there’s been almost no progress in tackling it – tumours can often be inoperable and treatment options are limited to chemotherapy and radiation. For the last 30 years, the five-year survival rate has remained stubbornly low at just 22 per cent.
“For a child with an inoperable tumour in their brain stem, they will be dead within a year, and radiation may only give them an extra month or two. It is horrific,” says Dr Jenkins.

Health & Medicine
Putting cancer cells to sleep
Dr Jenkins, a descendent of the Gunditjmara nation of western Victoria, heads a research team at the Walter and Eliza Hall Institute for Medical Research who are working on revolutionary forms of cancer treatments that involves manipulating our immune system to better fight cancer.
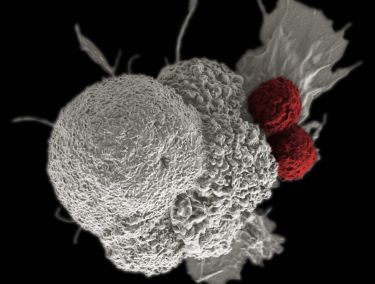
Specifically she is genetically re-engineering the killer white blood cells of our immune system, our killer T-cells, to help them recognise and attack cancer cells in the brain.
The problem is ensuring healthy cells aren’t attacked too, because in the brain any collateral damage is likely to have dire consequences.
Immunologists internationally are making huge advances in using microbiology to make our immune system more effective at fighting cancer, which is an expert at hiding from our T cells.
Last year, the Nobel Prize was won by immunologists for their discovery that proteins called “check point inhibitors” that stop our immune systems getting out of control can be manipulated, or switched off, to target cancer.
But not all cancers can have an effective immune response mounted against them in this way.
In addition, the critical nature of the brain makes tackling brain cancer particularly difficult and complex.
“Cancer originates from your own tissue, it isn’t an invader, and that makes it very hard for our immune systems to recognise cancer and attack it,” says Dr Jenkins, who is an honorary research fellow at the University of Melbourne.
Health & Medicine
The blood test tracking down microscopic cancer DNA
“Because cancer cells arise from your own cells, it makes it difficult to design treatments that can attack cancer cells without also attacking your healthy cells.”
Dr Jenkins’ key area of expertise is in engineering chimeric antigen receptor (CAR) T-cells. Receptors are the tiny antennae-like arms on the surface of cells that T-cells use to attach and break into cancer cells, much like a key opens a lock. Unless they have the right “key” a T cell won’t be able to kill a cancer cell.
“We are genetically engineering killer T cells to give them the precise key that can fit the lock of a cancer cell so that it can be killed.”
But making genetically engineered receptors is not an easy feat – there is a lot of sophisticated manipulation of DNA, which gets ‘cut and pasted’ using enzymes to make a virus which then infects the T cells to deliver the right ‘key’ to fit the cancer ‘lock’,” she says.
The “cutting and pasting” is all done in test tubes where fragments of DNA are recombined to form a receptor with all the specific parts needed to open the lock on a cancer cell. The new DNA is then transferred to T-cells harvested from the patient’s own blood by infecting the T-cells with a virus carrying the modified DNA.
But because Dr Jenkins and her team are dealing with the brain, they need to make the receptor highly specific or risk the modified T-cells attacking healthy cells.
“When we design receptors we have to make sure they only bind to the tumour cells and not the healthy cells. This is why what we are trying to do is so complicated. The receptors need to be exquisitely brain tumour-specific.”
By contrast, researchers using CAR T-cells elsewhere in the body can afford some significant side effects. For example, Dr Jenkins says one of the most effective CAR T-cells so far developed is able to successfully target a particular leukaemia blood cancer.

Health & Medicine
Backing the strengths of Aboriginal young people
But it comes at the cost of permanently damaging the immune system, meaning the patient needs regular injections to maintain antibodies which are important to keeping us healthy.
But collateral damage like this simply can’t be sustained when it comes to the brain.
“I think we have chosen to work on the most complicated problem we could have found,” says Dr Jenkins, but three years into the work she has no regrets. Her team have worked out how to kill brain cancer in a dish and will soon move into pre-clinical trials.
And the horrifying mortality from brain cancer is motivation enough for the team to keep going.
Three years ago, Dr Jenkins was working on general CAR T-cell development when a neurosurgeon girlfriend told her how distressing it was having to regularly tell people with brain cancer that their tumours were inoperable and they were going to die.
“When are you going to do something about that?” her friend asked her and her lab team.
“When we realised how devastating brain cancer was, we were motivated to use our expertise to a crack a problem to hopefully save lives.”
But the problem wasn’t just the challenge of working on the brain. Like all rare forms of cancer, brain cancer has long struggled to attract research funding, unlike a more prevalent cancers like breast cancer.
Health & Medicine
The cells giving our immune system more punch
“More research means better outcomes for patients and breast cancer is a good example,” says Dr Jenkins.
“Breast cancer is very prevalent so almost everyone knows someone with breast cancer, and that has helped support research. While 30 years ago the five-year survival rate was 70 per cent five years after diagnosis, it’s now 90 per cent.
That is the result not just of education and early detection, but also the investments in research.”
Funding for brain cancer research is being boosted through the Federal Government’s Australian Brain Cancer Mission established in 2017. In the latest funding round from Cancer Australia, Dr Jenkins, whose team works in collaboration with Professor Anthony Purcell at Monash University, was awarded a $A592,000 grant.
“We’ve now built up a head of steam and established an amazing team so it is now a matter of pumping through the data, but there is every reason to be optimistic.
“And the bottom line is that we need to make this work, because at this stage there is no other hope. Existing treatments are completely inadequate, but immunotherapies have the potential to offer real hope to brain cancer sufferers and their families.”
Banner: Glial cells that surround nerve cells, cultured from brain cancer stem cells. Steven Pollard/Wellcome Collection