
Sciences & Technology
The genes that turn malaria into a killer
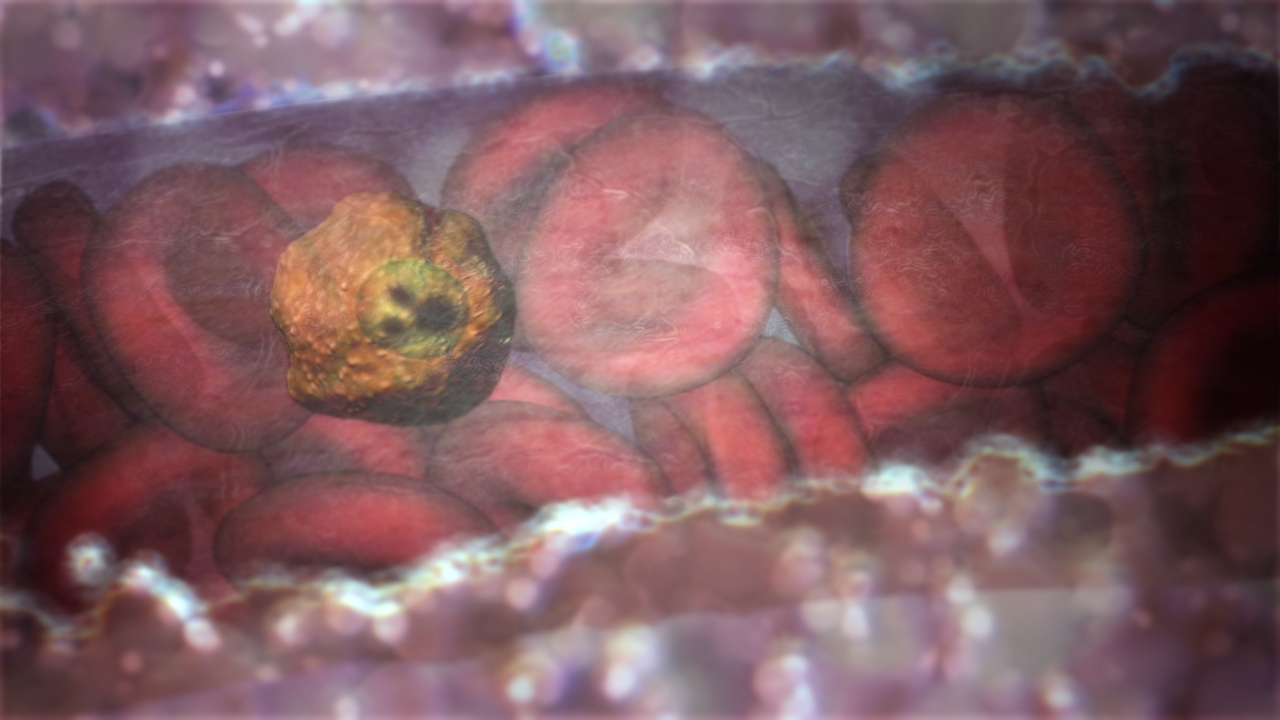
Scientists have used atomic level imaging to reveal for the first time exactly how an important malaria strain invades the blood, providing a design guide for a vaccine or drug
Published 28 June 2018
The mosquito-borne malaria parasite is an ancient killer, predating the emergence of humans. It has stalked us our entire history, possibly killing Alexander the Great and even being implicated in the fall of Rome. Today it still kills over 400,000 people every year, mostly children under five.
It has had thousands of years to get to know us, adapting to avoid our immune system in a way that modern biology is still struggling to unravel, making it difficult to develop new vaccines. But researchers have now deployed the most advanced imaging technology we have against this ancient parasite and revealed, at an atomic level, just how a major form of malaria (P. vivax) invades our red blood cells.
P. vivax is the most prevalent form of the malaria parasite, and is found mostly in the Americas and Asia, while the other major form, P. falciparum, is most prevalent in Africa. It enters the body through the bite and saliva of an infected female Anopheles mosquito. Once inside it travels to the liver where it invades cells and grows. In the case of P. vivax, this dormant liver phase produces no symptoms and can last or months or years, making it difficult to diagnose. When it finally leaves the liver and invades young red blood cells it triggers symptoms like fevers and shakes that can be fatal. There is as yet no licensed vaccine for P. vivax.
In results published in the journal Nature, the researchers used Cryo-Electron Microscopy (Cryo-EM) to expose the molecular structures the parasite uses to latch on to young red blood cells, precisely indicating the exact shape required for an antibody to bind to and stop the parasite from entering. An antibody is a protein produced by infection-fighting white blood B cells to help them attack specific pathogens, and one tailored to keep the parasite out of our red blood cells could be the basis an effective vaccine or drug.
A malaria parasite stranded outside a blood cell is easy prey for our immune system.
“The atomic level resolution this imaging gives us, has demystified how the parasite gets into red blood cells and we now have a clear design strategy for developing more effective antibodies,” says lead researcher Associate Professor Wai-Hong Tham from the Walter and Eliza Hall Institute and the University of Melbourne. “It has basically told us how to do it.”
Cryo-EM is an imaging technique that has been decades in development, earning its three separate creators the Nobel Prize in chemistry last year. It makes use of electron microscopes that use beams of electrons to image single atoms and freezing samples so quickly that they are immobilised and retain their natural shape. Previously researchers relied on using x-rays beamed through biological specimens preserved in crystals to generate atomic images, but the crystallising process meant that some biological material couldn’t be imaged in its natural state and often, large biomolecules do not crystallise at all.

Sciences & Technology
The genes that turn malaria into a killer
In an earlier breakthrough reported in the journal Science in January, an international team of researchers led by Associate Professor Tham and including WEHI colleague Dr Jakub Gruszczyk, discovered that P. vivax invaded young blood cells by latching onto a receptor on the outside of the red blood cell membrane. The blood cell uses this transferrin receptor to absorb iron and transport it around the body. Only young red blood cells have these receptors. By informed trial and error, they were able to develop antibodies that they demonstrated could stop parasite entry into red blood cells.
But the problem is that P. vivax comes in many different strains. An antibody for one strain may not work against another. What the researchers needed to know was whether there was a key common mechanism that all varieties used to invade red blood cells. If they could find that, they could create an all-purpose antibody. That is where the Cryo-EM came in.

Together with their lead collaborator Zhiheng Yu at the Cryo-EM facility at the Howard Hughes Medical Institute in Virginia, USA, the research team was able to generate precise atomic images of what was going on, and eventually uncover three critical and common connections across P. vivax strains.
“With the cryo-EM images we have been able to tackle one of the hardest problems in combating P. vivax,” says Associate Professor Tham. “It has given us exactly the architectural information we need for an effective antibody and pushed forward the development of a vaccine or drug candidate.”

Sciences & Technology
Malaria’s dark secrets exposed by a simple glow
“Malaria has co-evolved with humans for a long time so it has developed some ingenious strategies to get into our blood cells. That is why it has proven so hard to crack. P. vivax is especially difficult because it is so genetically diverse.
“But what we’ve done now is to expose all its sites of vulnerability at this key point in its life cycle. And while we don’t yet have the antibody that can do this for all strains, we will.”
The research team comprised scientists the Walter and Eliza Hall Institute, the University of Melbourne, the Howard Hughes Medical Institute, the Bio21 Molecular Science and Biotechnology Institute at the University of Melbourne, the Wellcome Trust Sanger Institute, Cambridge, UK, and the Big Data Institute at Li Ka Shing Centre for Health Information and Discovery, Oxford, UK
Banner Image: Animation of the malaria parasite in the bloodstream. Picture: Walter and Eliza Hall Institute