
Health & Medicine
Stimulating the brain – without major surgery

Embedded electrical devices are increasingly treating chronic illnesses, but researchers are now seeking to record and interpret our own electrical signals to predict symptoms
Published 28 May 2019
Chemical electricity is how we move, think, and remember.
And increasingly, as technology miniaturises and computer power multiplies, it’s how we are treating chronic illness.
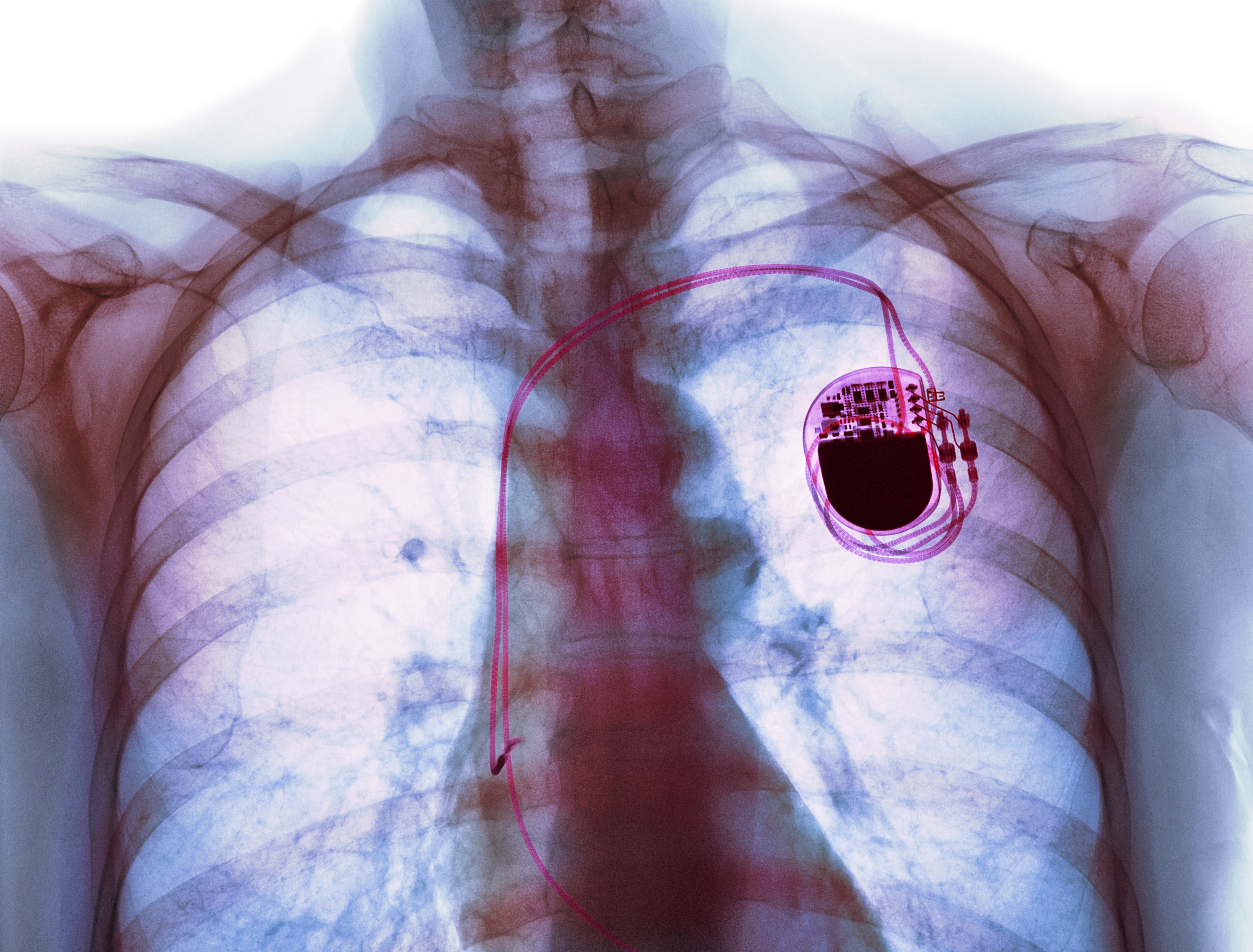
Since the fully implantable pacemaker was developed in the 1950s to keep a patient’s heart beating in rhythm using electrical impulses, engineers have now gone on to develop devices that can be implanted directly in the brain, under the scalp, or even inside blood vessels to treat diseases and disorders like Parkinson’s and epilepsy, as well as mental illnesses and paralysis.
But Professor David Grayden in the University of Melbourne’s Department of Biomedical Engineering is aiming to go further.
He and other researchers are attempting to listen back and interpret the body’s electrical signalling.
This would allow the new devices to actually predict the state of an illness or its symptoms – whether it’s detecting a looming seizure in the case of epilepsy, or the early stages of inflammation in a condition like inflammatory bowel disease (IBD).

Health & Medicine
Stimulating the brain – without major surgery
An electrical engineer and computer scientist, Professor Grayden tells me it’s all about “closing the loop”. He means going beyond devices that send out one-way electrical signals, to those that can record and ‘read’ the signals the body is sending back.
A feedback system like this, he says, could be tailored to an individual’s unique signal patterns, making it as efficient as possible in predicting when something is about to go wrong – triggering the device to respond and head it off.
“What we ultimately want to do is record and translate what an organ such as the brain is doing so that we can then stimulate it in a way to counter a problem before it becomes a problem,” says Professor Grayden, who holds the Clifford Chair in Neural Engineering.
Last year, in research published in the Journal of Neural Engineering, Professor Grayden and colleagues established that it was possible, in mice, to record and identify the precise nerve activity that results from inflammation in the gut.
Scientists have already shown that by electrically stimulating the vagus nerve, which connects the gut to the brain, it is possible to reduce inflammation in IBD.
But if scientists can detect the earliest signals of inflammation occurring, they may be able to prevent it from progressing.
“In any device for treating inflammatory bowel disease, closing the loop would be really useful because you could detect when inflammation has progressed to a set threshold that can trigger the device into action and suppress the inflammation before it takes hold,” says Professor Grayden.
Professor Grayden is well-suited to tackling the problem of reading the body’s nervous system, even though as an electrical engineer he had originally planned a career in the burgeoning telecommunications sector, not bioengineering.

Health & Medicine
Forecasting the cycle of epileptic seizures
It was his father’s own career as a linguist that led him to now deciphering the nervous system’s language, inspiring him take a course on linguistics alongside his engineering studies.
So, when he came to do his PhD, he put the two together and studied speech recognition technology. It was a unique skill set and when he graduated he was recruited to work on the development of the bionic ear and then the bionic eye.
Both rely on using electrical nerve stimulation and, importantly, being able to translate signals into sound and vision.
The body’s electrical signalling works at the atomic level and is chemically driven, but the model of how it works is similar to the way we use electricity to power our homes and cities.
A charge is created by an imbalance of ions, and these charged atoms pass through the walls of the neurons (nerve cells), transferring the charge within them.
Instead of copper wire that use electrons, the body’s nervous system conducts electrical charge using ions, mainly potassium and sodium ions, passing through the neurons.
“On the surface of neurons are proteins whose job is to pump ions in and out of the cells, creating a voltage across the cell membrane,” says Professor Grayden.
The voltage generated is usually only around 70 millivolts, or 70-thousands of a volt, compared with the approximate five volts used to charge a mobile phone.

“The charge is tiny, but then it doesn’t need to be large because it is occurring in a similarly tiny space – your nerve cells,” says Professor Grayden.
“It’s through these electrochemical processes that your brain works, including how we learn.”
“When you are listening or reading or seeing, you are actually causing voltage fluctuations across billions of neurons. And the amazing thing is that it actually affects your DNA and changes the structure of the neurons , adapting them to what you’ve now learned.”
It is these fluctuations in voltage that Professor Grayden is learning to read.
“The fluctuations in voltage the brain gives off are essentially electrical fields, like radio waves. So, in a sense, all these neurons in the brain are like little radio stations transmitting micro-voltage signals that can be picked up by a device and amplified.
“We can then have the same device respond to the signal by stimulating the brain where it is needed.”
“What we want to be able to do is record what the brain is doing and then stimulate it in a way where we can steer it to react in the way we want it to.”
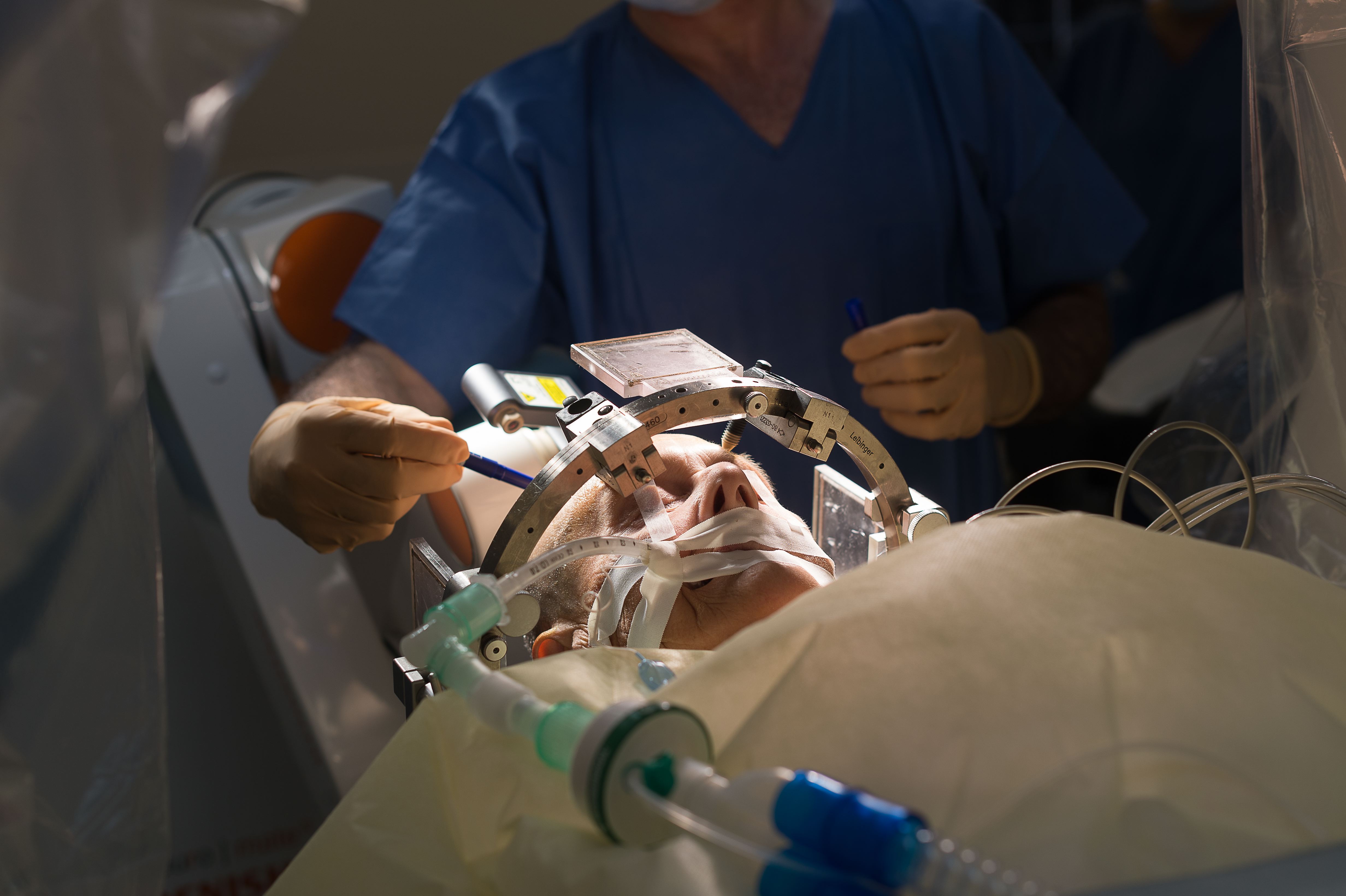
“In epilepsy, for example, there are devices that can be inserted under the skull or under the scalp that can constantly stimulate the brain to stop a seizure or detect that a seizure has started and stop it as soon as possible. But what we want to do is detect whether the brain is in a state where it is more likely to have a seizure and then stimulate the brain to stop the seizure before it starts.”
This also limits the side effects that can result when we stimulate the brain. One of the challenges when it comes to using electrical stimulation in the brain is that it isn’t necessarily precise. It means that stimulating the brain in one place can have ramifications beyond that area.

Sciences & Technology
Q&A: How algorithms are fighting epilepsy
“The stimulation can spread out from the area near the device and affect all the neurons in the vicinity,” says Professor Grayden.
For example, some of the possible side effects of brain stimulation include things like mood changes, confusion and difficulties with memory and thinking.
Closing the loop also creates the prospect of tailoring devices to individuals, so that they can be made more efficient by programming them to respond to the unique signalling of an individual’s body.
As wondrous as it is listening to Professor Grayden, I suddenly have an uncomfortable feeling that I’m just an electrical machine that is for now switched on, but, at some at point, will just switch off.
“What’s wrong with being a machine?” laughs Professor Grayden his engineer’s hardhat firmly on.
“I do struggle to see us as just being machines, but when you look at all the pieces of your nervous system, it really does look like a soft, wet and fragile machine.”
Banner: Getty Images