Health & Medicine
Watch Episode 6: Life Beyond Coronavirus: The Expert View
As scientists around the world continue to work on a COVID-19 vaccine – it’s important to understand how and why they work at a cellular level
Published 25 August 2020
This article is the latest in a series of weekly columns about all things infection and immunity written by Nobel Laureate Professor Peter Doherty for the Doherty Institute, Setting It Straight.
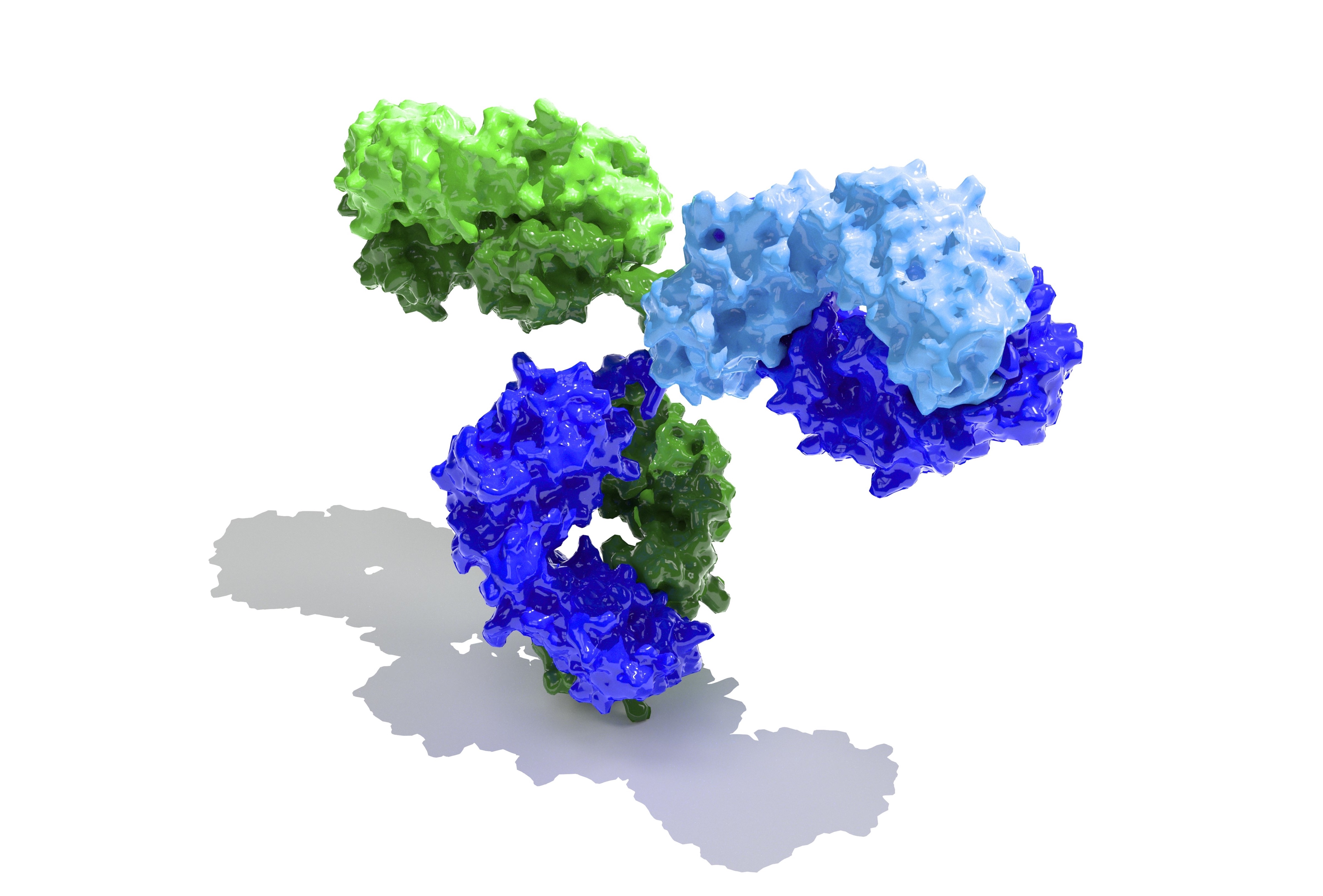
Over the past two weeks, we’ve looked at the basics of antibody (Ig) recognition and reflected a little on ways that the Y-shaped IgG molecule does its job of dealing with SARS-CoV-2.
Most equate being vaccinated with a jab in the arm and the ‘product’ going in by what is essentially the intramuscular (IM) route. The intent of that means of delivery is to establish a polyclonal (see Antibody dreaming) antibody response that provides the highest possible concentrations (titres) of virus-specific, neutralising IgG molecules in our blood.

Beyond that, as part of our overall immune memory to pathogens, the aim is that those antibodies will continue to circulate through the veins and arteries of our vasculature in the long term.
Given that the titres are high enough, some of that IgG will make its way out of the blood capillaries and into the fluids that bathe the cells of our respiratory and gastrointestinal tracts.
There’s also a different type of Ig, IgA, that has what’s called a secretory piece it picks up from cells in the mucosal epithelium as it passes through into the lumen of the gut and the lung, or nose.

Health & Medicine
Watch Episode 6: Life Beyond Coronavirus: The Expert View
If we want to induce virus-specific IgA, which will likely be produced by plasma cells that have localised to those mucosal epithelia, we need to deliver our vaccine intranasally (with a puffer), or orally.
Think of polio vaccines.
The original ‘inactivated whole virus’, Salk vaccine is injected and induces an IgG response. The live-attenuated (weakened) Sabin vaccine given as a drop on a sugar cube will multiply a little in the cells of the gut and induce both an IgG and IgA response.
The attached secretory piece can, for example, help protect IgA against degradation in the acidic environment of the stomach. In Australia, most of us are familiar with the jab (a protein subunit or inactivated virus) associated with influenza vaccination.

In Russia, Europe and the USA, children may receive a live-attenuated virus vaccine given by nasal spray which, again, has the potential to establish local IgA production.
Both IgG and IgA do essentially the same job when it comes to protecting us. With COVID-19, virus-specific Igs bind to three-dimensional ‘conformational determinants’ (antigens) on the SARS-CoV-2 spike protein and stop it from attaching to the ACE2 protein on our cells.
The consequence of such interference (stochiometric inhibition) is that the virus/ACE-2 complex is not pinocytosed (taken into) our susceptible nose and lung epithelia where, with the outer protein coat discarded, the viral RNA begins the process of exploiting the infected cell’s ‘molecular machinery’ to convert it into a virus production facility.

Health & Medicine
Lessons for a future pandemic
Blocked by the attached Ig and held in fluid phase (mucus or blood), the virus/Ig complex may instead (as described last week) be taken up (phagocytosed) by ‘big-eater’ macrophages and blasted to bits.
Another possibility is that ‘destroyer’ molecules of what’s called the ‘complement cascade’ may ‘find’ the virus via the Ig that labels it as surplus to requirements. I’ll leave that complicated story for the moment. We may come back to it in a later essay.
The takeaway message is thus: that the primary job of Igs specific for the SARS-CoV-2 spike protein is to ‘discover’ free, intact virus particles that have either come in from outside (in aerosols and/or droplets) or have been produced by infected cells within us.
The former is the major focus for vaccine development, the latter is part of the recovery process if, as immunologically naïve (unvaccinated) individuals, we do contract COVID-19 and then develop our own IgG and IgA responses.

Apart from infecting more and more cells within the respiratory tract, we know that SARS-CoV-2 enters the blood and, by what we call ‘systemic spread’, disseminates to other body organs like the heart and kidneys.
Once we are making enough of our own antibodies, that process should stop.
That brings us to an important difference between vaccination and infection. If the sole SARS-CoV-2 component in our vaccine is (as with the University of Queensland [UQ] ‘protein-clamp’ product) the appropriately folded spike protein, then we will only make antibodies against ‘conformational determinants’ on the spike.

Health & Medicine
Peter Doherty, 20 years after the Nobel Prize
The same will be true of, for instance, the US Moderna mRNA vaccine that provides the relevant genetic information so that we make the SARS-CoV-2 spike protein within us.
Of course, when it comes to the disease we call COVID-19, infection with SARS CoV-2 will, due to successive cycles of replication, produce a whole lot more spike protein than any vaccine.
That’s good? Maybe, but it’s not the whole story.
As some virus particles are constructed imperfectly, or degraded in various ways, we’ll also make masses of antibody against other virus proteins. If these are not expressed on the surface of the virus, or on virus infected cells, the Igs that are specific for these virus components will be of zero value for either recovery or to block possible reinfection.
As we’ll discuss later, some small components of those proteins may, however, be important in T cell–mediated immunity.
Still, from the aspect of our making the SARS-CoV-2 spike-specific Ig molecules that ‘grab’ an incoming virus particle before it does damage, a vaccine-induced response will be much more precisely targeted than that resulting from natural infection.
Banner: Getty Images