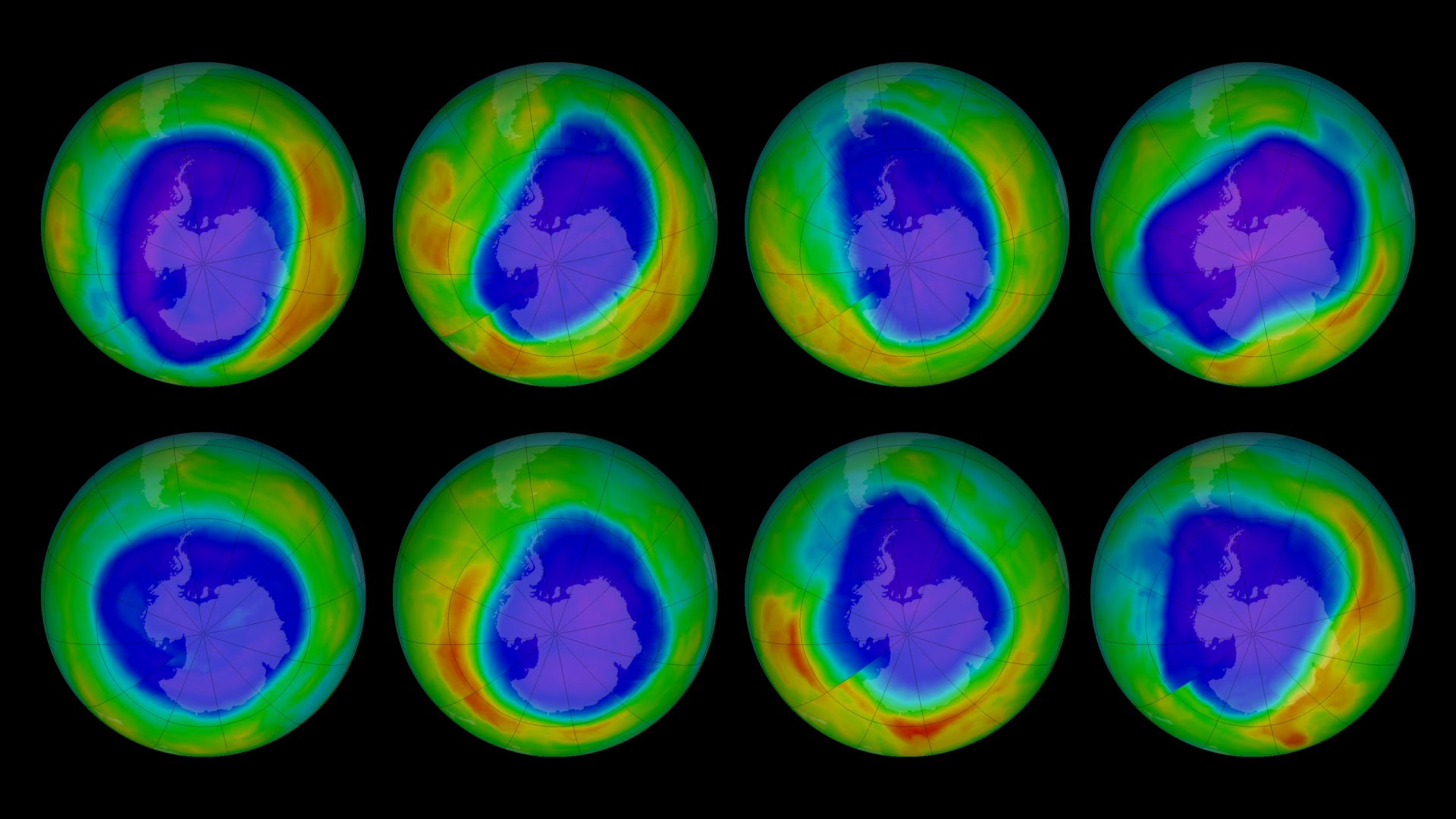
Whatever happened to the ozone hole?
Lessons in timely action to avert global disaster
Published 5 January 2016
Episode 333
In this Up Close podcast, atmospheric scientists Professor David Karoly and Dr Robyn Schofield from the University of Melbourne discuss the hole in the ozone layer over the Antarctic, and what effect timely global action taken in 1987 seems to have had in reversing ozone degradation.
Subscribe to Up Close through iTunes.


