
Sciences & Technology
Global food security under threat from crop and livestock diseases

Animal manure is a known source of antibiotic resistance genes, and now new research shows how these genes travel from the soil to a salad
Published 8 December 2019
The way our food travels from ‘paddock to plate’ is a journey that many people have become interested in over the past few decades.
Modern farming systems provide us with a reliable and predictable source of meat and vegetables, that help support population growth as well as our wellbeing.

But we are still trying to balance these positives with major issues like the impact on the environment and the use of antibiotics in livestock.
Antibiotics are medications that kill the bacterium or slow down bacterial growth. Since their introduction in the 1940s, antibiotics have become central to modern healthcare.
From the 1950s, antibiotics were approved by the FDA (Food and Drug Administration, USA) for use in food animals as growth promoters, as well as to prevent and treat infections.
Australia has strict veterinary prescribing guidelines where all antibiotics for animal use are evaluated and registered by the Australian Pesticides and Veterinary Medicines Authority (APVMA).
But even so, the spread of antibiotic resistance has become a 21st century public health concern across the globe.
Alongside the increasing consumption of antibiotics, veterinary antibiotics are poorly digested in the guts of animals, and a significant proportion of them (up to 90 per cent) are actually excreted into the environment.

The problem is that when livestock ingest antibiotics, the bacteria can mutate their DNA to survive, developing antibiotic resistance genes (ARGs).
These genes code for compounds that inactivate the antibiotic so the bacteria can survive, or stop the antibiotic entering the bacterial cell at all.
Bacteria can also share antibiotic resistance genes with microbes in the environment by swapping ‘mobile genetic elements’ (MGEs). These are a type of genetic material that can move around within a genome, or that can be transferred from one species to another.

Sciences & Technology
Global food security under threat from crop and livestock diseases
This becomes a major problem when ARGs move into disease-causing bacteria in humans, compromising the effectiveness of our current medical antibiotics. It is a problem compounded by the alarmingly slow rate at which we are discovering new antibiotics.
Although the emergence of antibiotic resistance is an inevitable consequence of the evolution of microbes as they adapt to different environments, human activities like the use of antibiotics in livestock production and the subsequent use of animal manure are considered one of the major driver behind the rapid prevalence and dissemination of ARGs, especially in the agricultural ecosystem.
Our previous research found that the application of animal manures as organic fertiliser enriched a large number of ARGs in agricultural soils.

Manure-derived ARGs have a high risk of spreading into the food chain and pose a potential threat to public health when vegetables grown in manured soils are consumed by humans.
But the actual transmission pathway of ARGs from manure to vegetable still remains unclear.

Sciences & Technology
Nitrogen pollution: Forgotten element of climate change
To better understand this, we established a glasshouse pot experiment with lettuce grown in soil with poultry or cattle manure. We explored the effects of manure application on the patterns of ARGs in soil and lettuce.
After 90 days of cultivation, we collected soil and lettuce tissue samples and performed molecular biology analyses to identify ARGs occurring in different areas – in the soil still attached to the lettuce root, inside the lettuce root itself, inside the lettuce leaf, and on the surface of lettuce leaf.
The application of manure, especially poultry manure, significantly increased the diversity and abundance of ARGs in soil and lettuce tissues.
Many ARG subtypes were also detected in the manured soil, inside the lettuce tissue, and outside of lettuce tissues, telling us that ARGs in manured soils are potential sources of ARGs in vegetables.
In this study, we proposed, for the first time, two possible transmission routes to predict the potential transfer of ARGs from manured soils to different compartments of the lettuce.
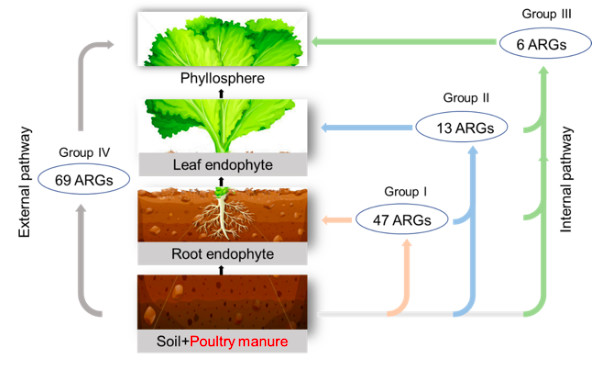
The first is the internal pathway. This is when ARGs transfer from soil into plant issues and colonise as bacteria or fungus (endophytes) within the lettuce tissue. The second, the external pathway, is when ARGs transfer from soil to plant surface through aerosol particles on leaf surface.
Our latest findings show that application of manure can result in a significant increase of ARGs in root endophyte, leaf endophyte and the above ground portion (phyllosphere) of the lettuce.

Environment
We can have urbanisation and enough to eat
We identified two potential transmission routes of ARGs from manured soil to vegetables, and highlighted the potential risks of environmental ARGs migration into the human food chain.
Now, it’s important to look at strategies to reduce the spread risk of antibiotic resistance from farm to fork for food safety and human health.
A reasonable use of antibiotics in livestock industry can ease this trend from the source, by initially decreasing the antibiotic resistance in the animal manure.
Raw manure disposal before agricultural application, like aerobic composting and anaerobic digestion, may also help to decrease the abundance of ARGs.
Most importantly is timing.
Manure application should be completed 120 days prior to harvesting of vegetables, which can allow the antibiotic residues, antibiotic resistant bacteria and ARGs in the manure to decrease before the produce is picked.
And last but not least, something we can all do at home (and many of us don’t) is wash our vegetables carefully, before they make it to the dinner plate.
This research is supported by an Australian Research Council Discovery Project led by Professor Jim He.
Banner: Shutterstock