Sciences & Technology
Engineering COVID-19 protection

Chemical engineers have developed a first-of-its-kind sensor that could boost low-cost medical and environmental diagnostic testing in developing and remote areas – all without the need for a lab
Published 27 April 2021
For most medical doctors, chemical and environmental engineers as well as forensic scientists, running a test is one of the first steps to diagnosing a disease, soil contamination or other issues.
Most tests are based on the principle that a chemical or a disease-causing agent – like a virus – will react with a reagent in a test to reveal its presence.
But these tests usually require the support of a laboratory and its staff, so what do you do if you don’t have access to these?

Our team of chemical engineers at The University of Melbourne has developed a device – a sensor the size of an Australian 50 cent piece – that could provide the answer.
The principle behind it may sound counterintuitive, as the device works by performing chemical reactions where the reactive materials are completely isolated from each other. The concept hasn’t been considered in the past, so this is the first prototype of its kind.

Sciences & Technology
Engineering COVID-19 protection
And while our discovery is exciting from a scientific perspective, perhaps more importantly it has led to a proof-of-concept sensor, now published in the journal Material Horizons that could be used for low-cost diagnostic tests that don’t need to be sent to a lab.
This could mean better care for people living remotely or in developing nations.
The key lies with individual carbon nanotubes made from the same material as pencil lead, that are one thousand times thinner than a human hair. When these nanotubes are placed together in a ‘mat’ to form a dense network, they can pass a charge between isolated chemical reactions in both directions.
When used in a sensor, this mat keeps the analyte – the substance being analysed – completely separate.
Depending on the test, the analyte could be a toxic gas, bacteria, viral particle, or even just water. By keeping it completely separate from a colour readout allows for detection at lower concentrations, and without contaminating the readout, which means the sensor can be reused.
This also means, for example, that it could be used for blood analysis, using very small amounts at the point-of-care.

Finding these reactions could occur when the test components are completely isolated from one another was entirely unexpected.

Sciences & Technology
A solution to engineering energy-saving smart materials
Normally, when an analyte comes into contact with a sensor it either binds to, or reacts with, a target sensing molecule – for instance, the older breathalyser systems or blood glucose monitoring devices.
This interaction typically leads to an irreversible reaction producing a colour charge that can be measured.
In our newly developed system, the reaction proceeds without direct contact between the analyte and the target sensing molecule, providing the same colour change but in a reversible way.
Normally the design of these isolated reactions, like those in a classic lead-acid car battery, would be thought of as an incomplete cell, with no way for charge transfer in one direction to be balanced out.
This ‘balancing’ is typically done with a simple salt bridge, as shown in the battery diagram below – which lets ions (a charged atom or molecule) move between wells to balance the electron motion.
Our breakthrough lets us do away with this ion motion, with all charge transfer occurring through our carbon nanotube mat instead of the bridge.
This discovery has a huge potential impact in the broader chemical and electrochemical fields, offering uses in chemical and environmental engineering, medicine and forensics.
So, we set out to meticulously investigate and prove the mechanism enabling these reactions.
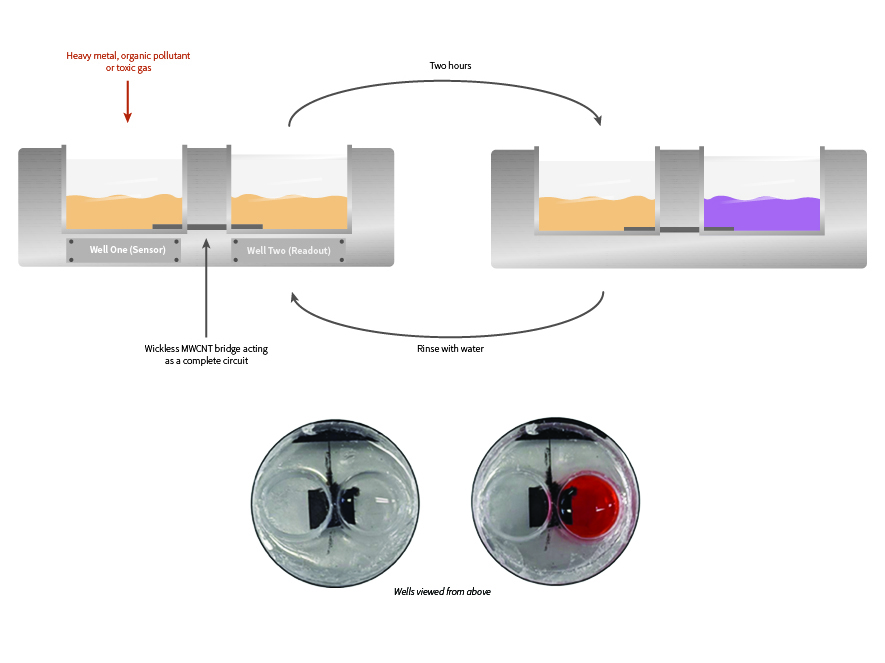
To do this, we made a cheap carbon nanotube mat and embedded it in a silicone matrix (the same material you would use as sealant in your bathroom) leaving just the opposing edges of the mat exposed to the environment.
By placing a chemical that would spontaneously decompose on one edge, and a chemical that changes colour when it reacts on the other (Cytochrome C, containing the same functional groups that make your blood red when it reacts with oxygen) – it was immediately apparent charge was moving without a salt bridge.
Sciences & Technology
The nanowires building greener nanodevices
To prove this result was entirely due to the unique properties of the carbon nanotubes, we tried to repeat our results with a solid gold mat – which doesn’t react with anything and is highly conductive.
Nothing happened.
Carbon nanotubes work because they are ‘ambipolar’; they can pass both positive and negative charge easily through their structure. When organised in dense networks of conductive paths, charge can be balanced and move freely between the two isolated chemicals.
This new mechanism and sensor device is exciting because it no longer requires external energy or the mixing of chemicals for a reaction to occur.
By using chemical energy instead, it can be performed anywhere and at any time.
We think it will be particularly useful for biosensing; detecting biologically relevant molecules, like hormones or disease markers.

The device uses a colourimetric readout, which just means a clear colour change, letting anyone with an instruction booklet interpret the result with little-to-no training.
Colourimetric readouts are already common in one of the most ubiquitous sensors globally – the pregnancy test.
Sciences & Technology
Wearable devices that use human energy
The ability to simply and clearly see a positive result also allows the elimination of expensive detection equipment. This could be particularly impactful for developing countries where electricity is sparse and well-equipped hospitals far away, making point-of-care detection vital.
Cutting down the time and training needed to detect disease, or even a virus outbreak, could transform outcomes, reducing illness and, even, mortalities.
While we have demonstrated the new mechanism and made proof-of-concept devices, we’re just at the start of this exciting project.
The next steps are to dive into specific detection challenges that currently need a simpler and cheaper solution, like environmental monitoring of remote waste sites and disease tracking in remote communities.
There is a long road ahead but with this discovery – cheaper, quicker and easier sensing technology is on the horizon.
Banner: Getty Images