
Sciences & Technology
Probing Earth’s deep and ancient secrets

In the 90s, Professor Richard Robson created a new class of coordination polymers - now he and two other scientists have won the Nobel Prize in Chemistry
Published 15 October 2019
Unless you are a chemist you probably haven’t heard of MOFs, but they could soon have a significant impact on your life.
Metal-organic frameworks (MOFs) are a new class of solids that are being developed into gas and energy storage devices, catalytic reactors and other potentially revolutionary products.
Now, Professor Richard Robson is part of the group of researchers awarded the 2025 Nobel Prize in Chemistry.
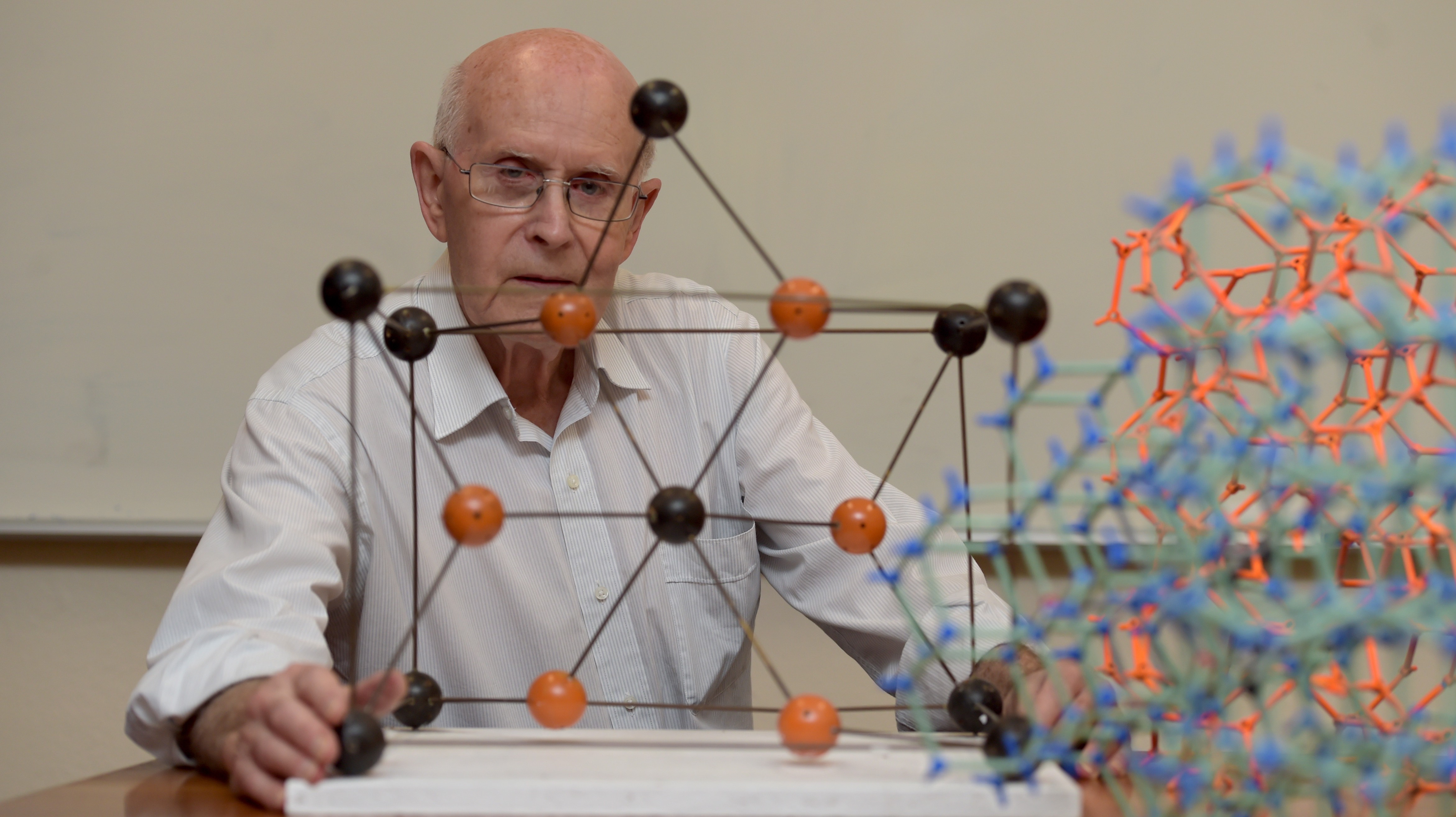
It all started with a chemistry lecturer, some wooden models, and an itch that wouldn’t go away.
In 1974, Professor Richard Robson, then a lecturer in the Department of Inorganic Chemistry at the University of Melbourne, was invited by his departmental head, Professor Don Stranks, to build large wooden models of crystalline structures for first year chemistry lectures.
Little did he, or anybody else, know that these models – sodium chloride, caesium chloride, fluorite, zinc blende, wurtzite, rutile and others – would inspire an entirely new field of chemistry.
To build these models, Professor Robson assembled wooden balls in different colours representing atoms (like sodium and chloride for a salt crystal), and connected them with rods at the correct orientation to replicate the atomic arrangement of the crystals.

Sciences & Technology
Probing Earth’s deep and ancient secrets
“So, what I had to do was to calculate the angles, which for me was something new entirely.
"The workshop would have to drill holes into wooden balls at these angles so that you could assemble the models, and I used trigonometric tables for that job,” says Professor Robson, now Professor Emeritus of Chemistry at the University of Melbourne.
When the balls came back from the workshop, Professor Robson was struck by the automatic nature of the assembly process, once it was underway.
“It became apparent that the balls were invested with information – they were pre-disposed to produce the structure that we were intending to produce,” he says.
“And that led to the thought: ‘What if you used molecules in place of balls and chemical bonds in place of rods?’”
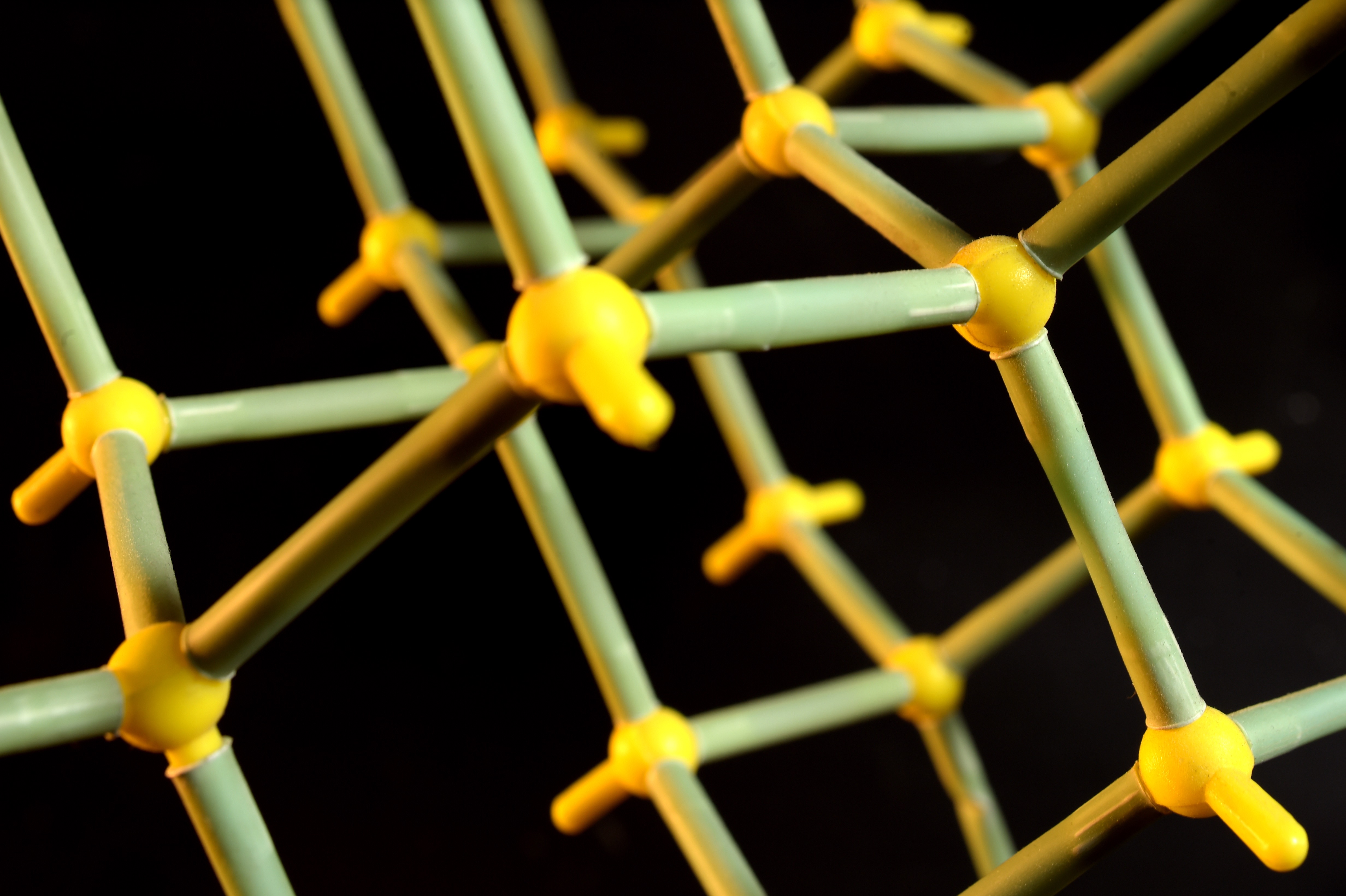
Each year, Professor Robson would bring out the models for the first-year lecture, and would think to himself, “that idea might have some merit”, and then pack the models away and get on with his teaching and research.
It took ten years before he finally started work at the bench to test his idea out.
To start, Professor Robson chose the simplest crystal structure he could think of – diamond.
In diamond, each carbon atom is connected to four others in a triangular pyramid, known as a tetrahedron. All carbon-carbon-carbon angles are 109.5 degrees.

Sciences & Technology
Smashing through science’s glass ceiling
“At that time any sensible reasonable chemist would have said our chances of creating a crystal were zero, that you’d get tangled bird’s nests – amorphous, nasty stuff that you couldn’t handle,” he says.
“It turned out that it worked marvellously well. And we did get crystals.”
The resulting substance, Cuٰ[C(C₆H₄.CN)₄]BF₄, did have the intended diamond-like connectivity, but even Professor Robson’s closest collaborator, Dr Bernard Hoskins, had serious reservations.
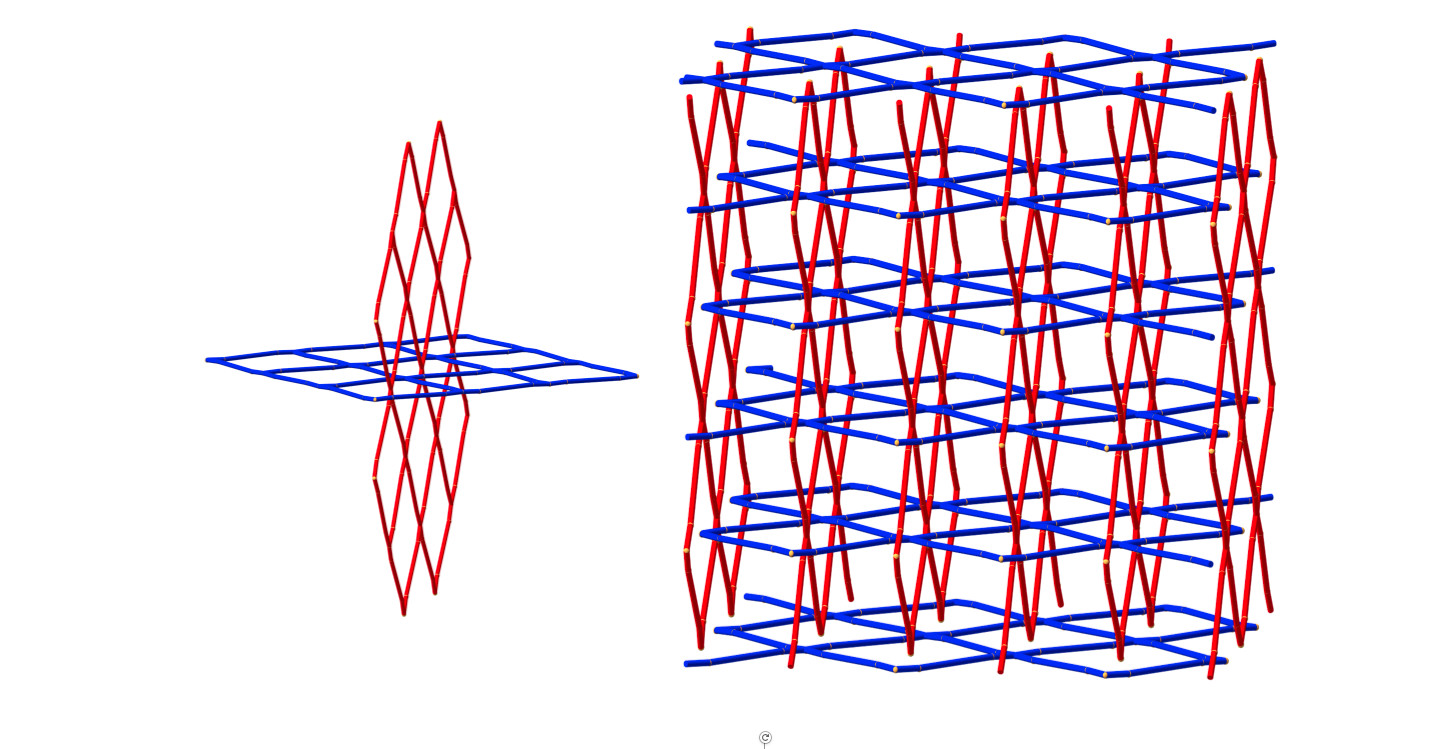
Standard crystals are very densely packed, with no gaps between the atoms, but this new material was different – more than half of its contents was liquid.

By replacing direct chemical bonds with molecular rods, Professor Robson had introduced something completely new into these crystalline structures – space.
“Bernard did the crystallography (proving that the substance had the desired structure) and he was very worried about all that space,” says Professor Robson.
In the early papers describing this new class of coordination polymers, Professor Robson laid out a series of predictions about these structures.
All of which have turned out to be true.
Among other things, he predicted that an almost infinite range of structures could be produced, that ‘open structures’ with large channels and cavities would be developed, and that the frameworks could be ‘functionalised’ with catalytic sites.
Health & Medicine
Surgeons go green: Recycling general anaesthetic
This meant, for example, a substance could flow through a framework and be chemically transformed on the way through.
“I think what we started to make obvious and possible, involved a thousand times more work than we ourselves could possibly do,” says Professor Robson.
The initial discovery was well-received, but didn’t immediately spark the huge interest that was to come; an explosion of interest that ended up diminishing Professor Robson’s part in its development.
In the meantime, Professor Robson went back to the lab and started exploring other kinds of building blocks.
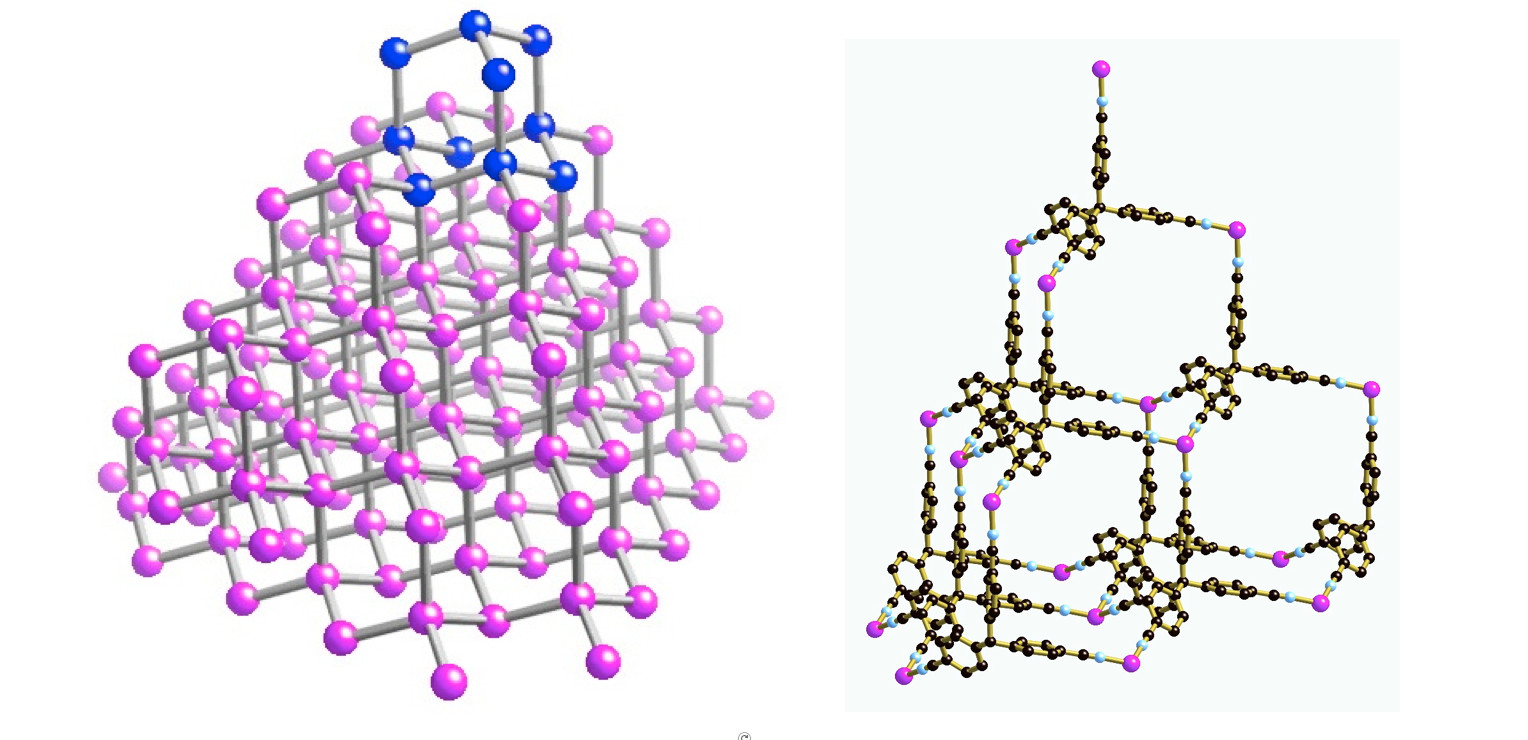
He took the known properties of metals and connecting molecules, or ligands, envisaged the structural form they might take, and then mixed them together, often with remarkable success.
In the early 90s, his group created a variety of building block geometries.
In the following three decades, they created an astonishing array of three-dimensional structures, including complex arrangements with multiple, interweaving frameworks.
Professor Robson and his colleagues attracted a lot of attention when they started seeing peculiar, and very beautiful, interpenetrating structures.

Sciences & Technology
Heroines of mathematics
“It’s interesting because it brings units from different systems into close proximity,” says Professor Robson, “and you may be able get interesting electronic interactions between them and throughout the network.”
In the mid-1990s, Professor Omar Yaghi, an influential chemist from the University of Michigan (now at the University of California) started working on similar structures, and soon came to dominate the field.
Professor Robson and his colleagues didn’t give their structures a formal name, which he maintains was unnecessary, as these structures were simply an application of the century-old field of coordination chemistry.
But in around 1994, Professor Yaghi did give them a name – Metal-Organic Frameworks, or MOFs for short – calling this field of research ‘reticular chemistry’.
But, in the past few years, Professor Robson’s work has begun to receive acknowledgement.
A turning point within the MOF community occurred in December 2018, when Professor Robson was invited to give a plenary at the 6th International Conference on Metal-Organic Frameworks – his first attendance at a MOF conference.
In his humble fashion, Professor Robson says he thinks it went “quite well”.
“They clapped,” he says.
Thousands of MOFs have been produced and there has been progress in developing some of the applications Professor Robson predicted in the early 90s.

Sciences & Technology
The dark matter detective
But large-scale commercial applications have yet to materialise.
Associate Professor Brendan Abrahams, a long-time collaborator of Professor Robson, says there was a lot of interest in on-board storage of hydrogen in cars, because MOFs could remove the need for heavy, high pressure hydrogen storage tanks.
Professor Robson has hopes that the next generation of high-temperature superconductors will be coordination polymers.
“We still haven’t been able to get things that conduct electricity well, but it’s just a matter of being able to make these compounds,” he says.
But Professor Robson is happy spending his time in the lab, designing and creating new and interesting structures.
“My contribution in all of this has been more like that of an artist or an architect,” he says.
“Highly non-scientific, in fact, and any scientific respectability that’s come out of it has been due to people like Brendan Abrahams, Bernard Hoskins, and Tim Hudson, who did the crystallography.”
University of Melbourne Professor Richard Robson is one of three scientists awarded the 2025 Nobel Prize in Chemistry.
This article was updated on 9th October, 2025.