
Sciences & Technology
Helping corals survive a rapidly changing world

The discovery of bacteria inside the algae that live inside corals sheds new light on reef-building corals and could be important for understanding coral bleaching
Published 11 March 2021
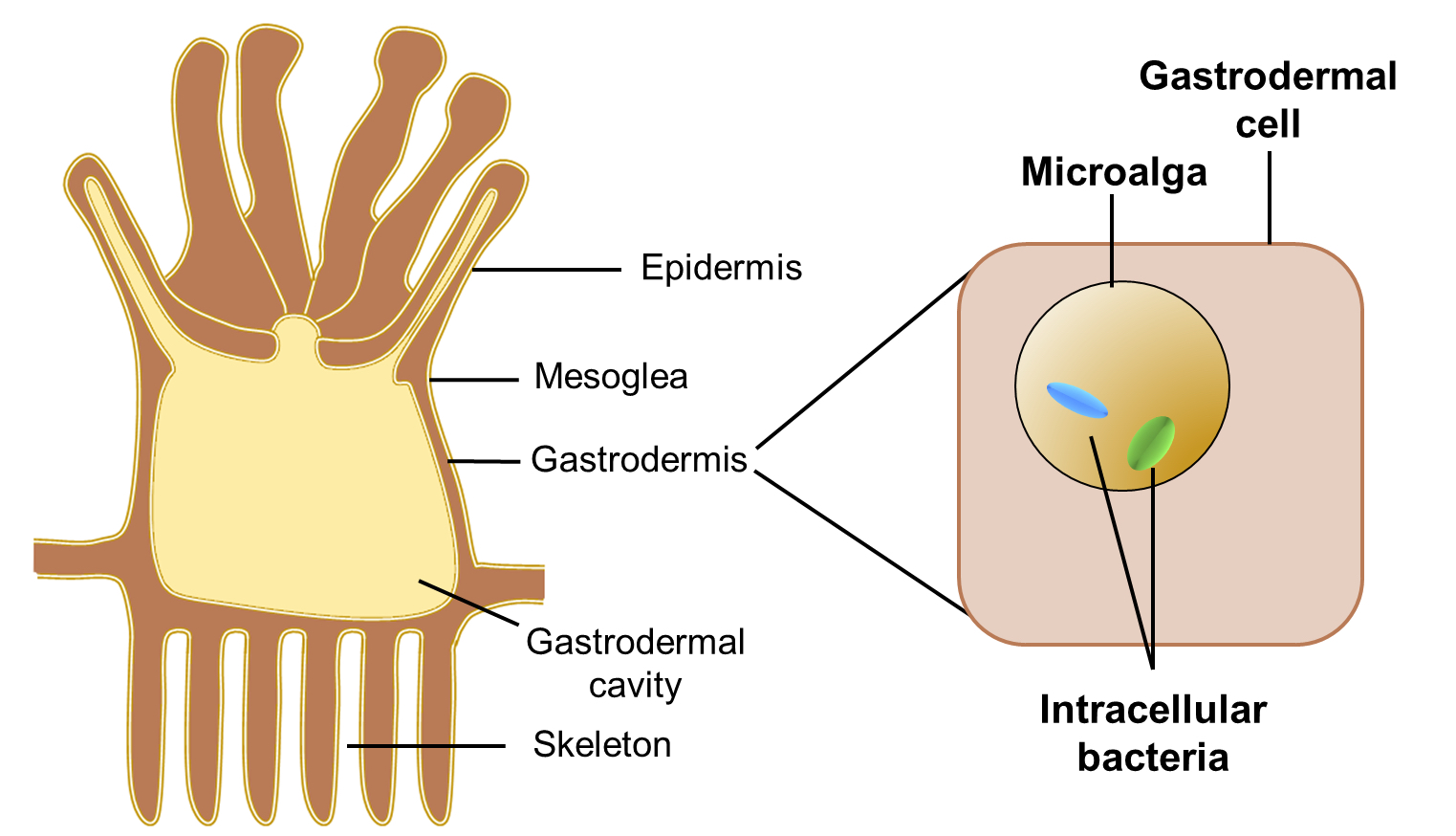
The ability of corals to build and sustain reefs is supported by tiny intracellular algae that live inside the cells lining their gut.
Our new research, published in the International Society for Microbial Ecology Journal, has found that these microalgae also have their own internal symbionts – that is, bacteria living inside their cells.

This is important because corals receive most of their nutrition from these microalgal symbionts. Alarmingly, this coral-microalgal symbiosis and the future of coral reefs is currently threatened by rapid climate change.
Armed with this new information about the bacteria inside these microalgae, our team at the University of Melbourne and the Australian Institute of Marine Science is exploring whether a bacterial probiotic cocktail can be developed to enhance coral thermal tolerance and ‘buy time’ for coral reefs until climate warming is properly addressed.
The intracellular bacteria harboured by the microalgae are diverse with the most abundant bacteria representing three families, but similar bacterial communities are found in the nine microalgal species we examined.

Sciences & Technology
Helping corals survive a rapidly changing world
While the functions of these bacteria are currently unknown, this conserved composition of bacterial communities present inside all microalgae that we have so far analysed points to roles that are important to the health and functioning of the microalgal hosts and the corals in which the microalgae reside.
With the help of the sun’s energy, the microalgae living inside corals make organic carbon molecules from carbon dioxide and water, through the process of photosynthesis. A large portion of these products of photosynthesis are translocated to the coral host tissues and, in this way, meet most of the coral’s energy demands.
The microalgae also increase the speed by which the coral deposits its calcium carbonate skeleton. This means that without the coral-microalgal symbiosis, there wouldn’t be any coral reefs.
The symbiosis is sensitive to periods of higher-than-usual sea surface temperatures, causing the loss of the microalgal symbionts from the coral tissues (what we know as coral bleaching), which is often followed by coral death.
The frequency, intensity and duration of summer heatwaves are increasing with global climate change and these extreme events have resulted in the death of more than half of the corals on Australia’s Great Barrier Reef since 2016.
It is well-known that most corals have a diverse bacterial microbiome, similar to the human gut.

Sciences & Technology
Breeding baby corals for warmer seas
Most of these bacteria reside in the mucus layer that covers the coral surface and in the coral skeleton, just beneath the coral tissue, but they can also occur within the gut and tissues.
However, compelling evidence for bacteria that live inside the coral or microalgal symbiont cells was absent until now.
Our study, in collaboration with the University of Technology Sydney, aims to determine the diversity and location of bacteria associated with microalgal symbionts. It’s possible to remove bacteria that are attached to the outside of the microalgal cells by briefly washing the cells in bleach.
This treatment only leaves the intracellular bacteria – those living inside algal cells – behind, which can be accessed by breaking the microalgal cells open, after which we can sequence their DNA.
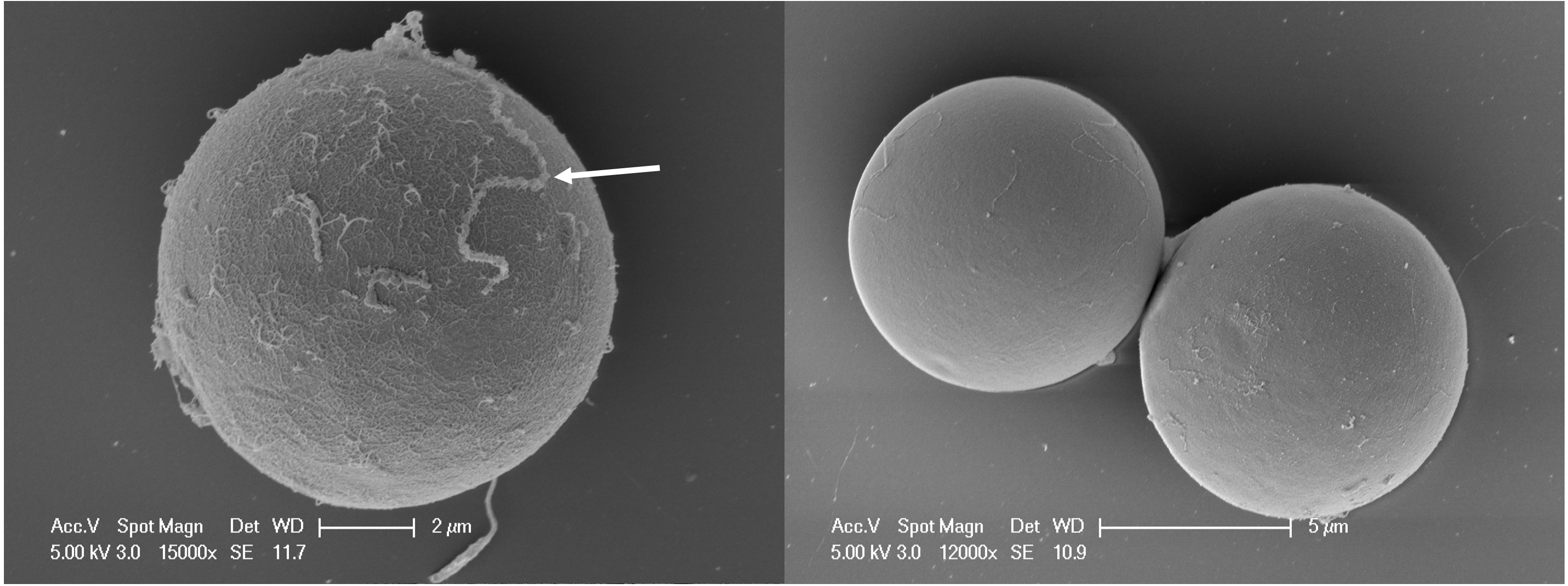
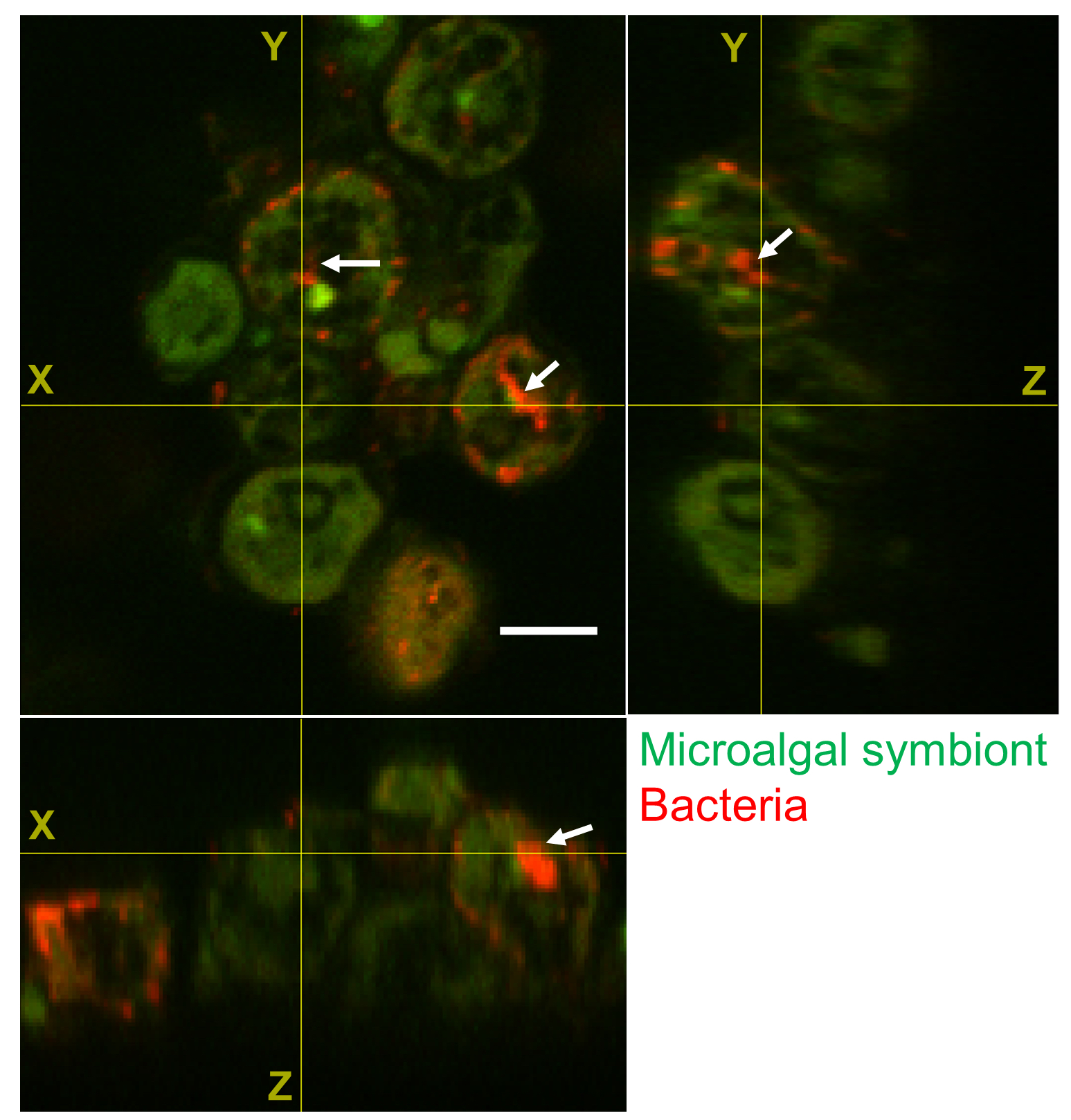
The location of the bacteria is then further validated by three-dimensional laser scanning microscopy.
With this method, bacteria are visualised by using a fluorescent probe that binds to parts of the bacteria and lights up when excited by a laser beam.
Different focal planes are scanned by the laser and later put together to reconstruct a 3D image of the microalgal cell and its associated bacteria. By combining DNA sequencing and microscopy methods, our research provides unequivocal evidence of the taxonomic identity and intracellular location of bacteria.

Sciences & Technology
On the hunt for ancient reefs
Our findings, funded by an ARC Laureate Fellowship and an ARC Discovery Project reveal that corals have many layers. For decades, the intracellular microalgal symbionts of corals have been the focus of hundreds of scientific studies.
We have now peeled away another layer of these complex organisms and revealed that – like nature’s Russian dolls – corals harbour symbionts within symbionts.
The microalgal symbionts largely determine the coral bleaching temperature tolerance, and our study suggest bacteria may play a role too. The next challenge is to decipher what this role is.
Our team is currently working towards obtaining full genome sequences of these intracellular bacteria; knowing which genes are encoded within these bacterial genomes will provide important information on their functions within the algal cells.
Bacterial gene expression analyses and metabolite mapping across the coral and its associated microbes will be used to assess the gene activities and location of metabolic products of the symbiotic bacteria. This will allow inferences of their interactions with their first level (microalgal) and second level (coral) hosts.
We are also attempting to grow the bacteria in the lab. If we manage to do this, we will try to experimentally evolve enhanced versions these bacteria and then reintroduce them into microalgae and coral to try and increase coral bleaching tolerance.
Our hope is that this work will contribute to helping coral reefs survive this century until climate warming is curbed, after which corals can recover naturally.
This study was co-authored with Sam Girvan, Sophie Barkla, Dr Alexis Perez-Gonzalez and Professor Linda Blackall from the University of Melbourne, and Professor David Suggett from the University of Technology, Sydney.
Banner: Getty Images