
Health & Medicine
Watch Episode 4: Life Beyond Coronavirus: The Expert View
Unless a new treatment is discovered, developing a vaccine is crucial to controlling COVID-19. In this special report, our experts explain the complex challenge of creating, testing and distributing a potential vaccine to end the coronavirus pandemic
Published 15 June 2020
The World Health Organization has warned it could take four to five years for the world to finally control COVID-19 – and it may never disappear.
“We do have one great hope; if we do find a highly effective vaccine that we can distribute to everyone who needs it in the world we may have a shot at eliminating this virus but that vaccine will have to be highly effective.
“It will have to be made available to everyone and we will have to use it,” WHO’s Executive Director of Health Emergencies Dr Michael Ryan said recently, cautioning that an effective vaccine remains a “massive moonshot.”
Here, University of Melbourne researchers take us behind the scientific search for this “moonshot”, explaining how the virus and our immune systems work, how a vaccine is identified, developed and tested and – finally – how it’s deployed.
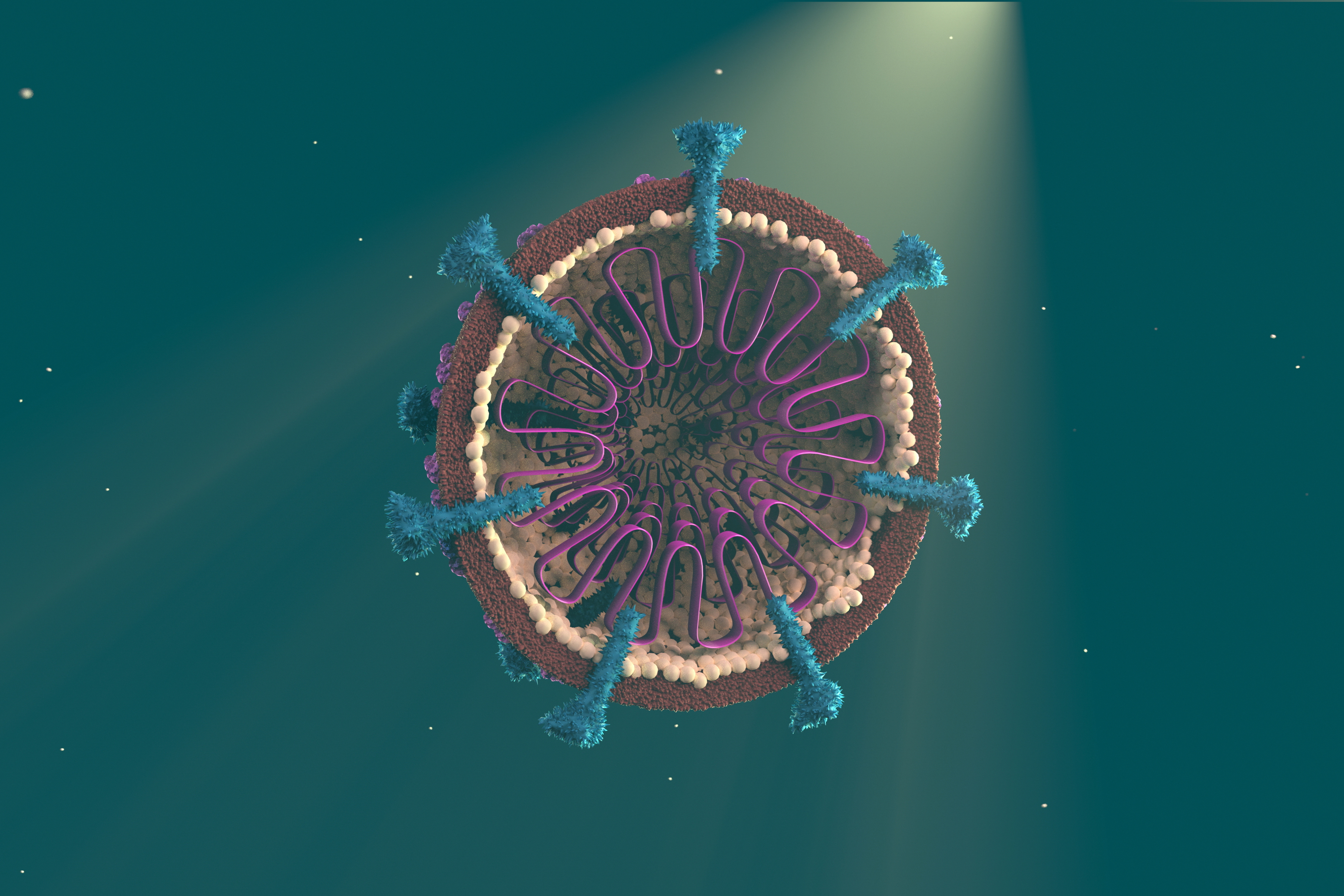
Viruses are tiny biological entities, much smaller than bacteria. While a bacteria may be made up of a few thousand proteins, most viruses amount to as few as 10 to 100 proteins.
And, unlike bacteria, viruses can’t reproduce themselves on their own. This is why they are not thought of as living things.
Viruses can only reproduce themselves by invading the cells of a host, like us, and that is what makes them dangerous.
Once inside the body, unless a virus can be quickly killed by our immune system, it will try to break into cells and hijack our proteins to replicate their own genome (the sum total of an organism’s DNA).
This is why it is difficult to develop therapies to kill viruses – antivirals.
We are able to target bacterial infections using antibiotics that attack the proteins in bacteria. But because viruses use our own proteins, antiviral therapies attacking these proteins can cause potentially damaging side effects.

Health & Medicine
Watch Episode 4: Life Beyond Coronavirus: The Expert View
It means that effective antivirals need to be specifically targeted so they attack only those proteins we know are critical for the virus. That way we can minimise any potential harm to the overall health of the patient.
Antiviral researchers also face the ongoing problem of resistance. Just like bacteria, viruses can develop resistance to antivirals over time.
The virus that causes COVID-19, known as SARS-CoV-2, is a type of coronavirus; these are named for the spikes on their surface that give them their crown-like appearance. They commonly attack our respiratory systems.
SARS-CoV-2 is named after the related earlier coronavirus that caused the 2003 outbreak of SARS (Severe Acute Respiratory Syndrome). But unlike SARS, SARS-CoV-2 has a few tricks up its sleeve that we believe make it more infectious and potentially more dangerous.

The ‘spikes’ on SARS-CoV-2 that it uses to break into lung cells, appear to be able to bind more efficiently to the outside of our cells, making it more effective at breaking in.
This means that people can be more easily infected with COVID-19 compared with SARS.
SARS-CoV-2 also uses our own enzymes to trigger its spike action, again making its spread more efficient.
Finally, SARS-CoV-2 causes less immediate symptoms once it has infected someone – meaning an infected person can initially appear asymptomatic, making it easier for SARS-CoV-2 to spread.
On the plus side, our research team showed in March that usually our immune systems are able to launch a robust response to fight SARS-CoV-2, which is good news for vaccine developers.
By tracking blood samples in a COVID-19 patient with mild symptoms, we were able to map how our immune system goes about fighting this coronavirus. We are now trying to understand which parts of the immune system ‘go awry’ in those who develop severe disease.
It means vaccine developers now have a range of areas in our immune system that they can potentially target in creating a vaccine that can head off infection or make it less severe.
The innate immune system is our first response to an infection, like a virus, and involves our defence cells – white blood cells – quickly going on the attack.
But it’s a generalised response and sometimes this isn’t enough to kill an infection.
That is when we need our adaptive immune system to kick in with an exquisitely specific response that precisely targets a virus.
It does this by recognising the specific structures, or antigens, on the outside of a virus or a virus-infected cell, which are then attacked – neutralising it.
A key part of this system are the white blood cells called B-cells. These cells are specialised from each other by having different receptors on their surface that recognise specific antigens.
These can be secreted as antibodies that can then bind to a specific antigen, marking a virus for attack.
Once the adaptive immune system recognises an invading virus, a network of chemicals and proteins finds and alerts B cells that have exactly the right shaped antibodies.
These B-cells then multiply and secrete copies of the correct antibodies that circulate around the body marking viruses and virus-infected cells that can then be killed by other cells in the immune system.
Health & Medicine
Watch Episode 2: Life beyond Coronavirus: The Expert view
The weakness of this adaptive system is that it takes time to mount its attack, usually about two weeks, by which time the body may already be infected.
This is where vaccines can help.
Once a virus is killed, the antibodies continue to circulate in the body. In this way the immune system remembers the virus. It means we are ready and armed to mount an immediate large-scale assault with the correct antibodies if the virus returns. And this is what we mean by having immunity.
Vaccines work by mimicking an infection, introducing to the body harmless parts of a virus – the antigens – which is enough to trigger the immune system to make the antibodies people need to be immune.
But vaccines aren’t usually completely effective in everyone.
This is partly because the immune system’s new defences triggered by the vaccine are based only on a part of a virus, not the whole thing.
However, the varied effectiveness is mostly because immune systems are different from person to person. Some people better produce antibodies than others and not everyone who is vaccinated will have immunity.
There are genetic factors at work here too.
We think it may be genetic that some young and healthy people are so severely affected by COVID-19 – they seem to be missing a key component specific to this virus.
Our team is working as part of the global COVID Human Genetic Effort to try and identify these genetic factors to improve treatments.
Part of my own work is focused on tackling disorders where people’s immune systems just don’t work properly.
Health & Medicine
Watch Episode 1: Life Beyond Coronavirus: The Expert View
One of the treatments we use in primary immune deficiency is giving people a serum made up of donated plasma rich in protective antibodies. A similar approach is already underway to collect and purify plasma from donors who have successfully recovered from COVID-19.
But this is expensive and the antibodies only live for around 20 days, which means people need repeated top-up doses.
A crucial question for any vaccine is how often you will need it.
At this stage we just don’t know how long immunity to COVID-19 lasts, but based on what we know about SARS it may be a couple of years.
Viruses are also mutating all the time. So far, COVID-19 is mutating relatively slowly and in ways that are likely to be benign.
It may be that in the future, if COVID-19 hangs around, a vaccine may need to be seasonal like the influenza. That could be our new reality.
The challenge we face now is that so far no one has been able to produce an effective vaccine against a human coronavirus – and the virus that causes COVID-19 appears to be a real battleship of a virus with many immune-evasion weapons on board.
We know from previous efforts to develop a SARS vaccine, that coronaviruses seem to be able to sometimes “distract” the immune system.
It means our bodies produce antibodies that attack the wrong parts of the virus, rather than the critical structures that the virus uses to break into cells and spread.
We suspect this may be why some cases of COVID-19 can suddenly become so severe. The immune system gets off track and non-protective antibodies help the virus spread through the body.
So we need to be careful that any vaccine isn’t likewise presenting immune system “distractions” of the virus.
We know that during tests of one failed SARS vaccine, antibodies that were initially considered useful towards the virus, instead quickly caused a very severe case of the disease when the vaccinated animals were challenged with infectious SARS virus.

Politics & Society
The World Health Organization as pandemic police?
Here in Australia, we think that the answer to avoiding the immune system getting distracted is potentially creating a vaccine that specifically zeroes-in on that most critical part of the virus needed to block infection.
In the past, vaccine development was about exposing the immune system to many parts of a virus. But most approaches now selectively present the immune system with only very specific parts of a virus to focus crucial antibody immunity.
In the case of COVID-19, we are targeting a spike on the surface of the virus that acts like a sort of spring-loaded box-opener that it uses to pierce and deposit the payload of virus genes into cells.
Once inside it can take over the cell’s genetic coding and reproduce itself while hiding from the immune system.
The team at the University of Queensland, who we are working with closely, are using a technique they developed to grow and clamp in place the spring-loaded spike assembly as a separate structure that they can then introduce to the body – looking like it’s fully-armed.
The aim here is to produce what are called ‘neutralising’ antibodies that prevent the ‘popping’ of the virus’ entry-machinery.
These antibodies are produced by the immune system only in response to this authentic spike structure, so we think the antibodies will be tailored enough to reliably attack, or neutralise, the virus right where we want.
At the Doherty Institute, we are taking a similar and complementary approach.
Health & Medicine
A new weapon in the war against superbugs
There is a risk that an immune system exposed to just the spike may end up producing antibodies that attack the wrong end of the spike – its base rather than its tip.
So, we are working on tethering a collection of spikes through their base onto a non-infectious ‘virus-like particle’ – made from a small amount of other virus support proteins.
This could give the immune system a better guide to producing antibodies that can attack the tip of the spike, where the box-opener sits, rather than the base.
It’s all about working at the molecular level to set our immune system on the right track from the start so that it only produces these neutralising antibodies, rather than ‘distracted’ antibodies that don’t hit the virus where it hurts.
According to the World Health Organization, more than 120 COVID-19 vaccines have been proposed, of which at least six are in clinical trials.
But we need to remember that creating a vaccine is complex and difficult. Even when we have something that appears to work it takes time to establish that a vaccine is safe to use in humans and is actually effective in populations.
And there can be many failures along the way.
For example, despite decades of work, we still haven’t produced vaccines for many diseases that researchers have focused on, like a vaccine for the HIV virus, or for the sore throat-causing Streptococcus-A bacteria that can go on to give rise to rheumatic fever and heart damage.
Neither do we yet have a universal vaccine for influenza.
That means new, or novel, strains of influenza can cause dangerous pandemics. The last time an influenza pandemic occurred was in 2009-10, when a new influenza virus emerged in Mexico. The one before that was the 1968 Hong Kong flu.

Health & Medicine
Lessons for a future pandemic
It just goes to show that is a persistent problem that we haven’t yet solved.
And safety has to be paramount.
We know that one of the previous unsuccessful attempts to create a vaccine for the SARS coronavirus appeared to make animal subjects more susceptible to severe infection.
Once a candidate for a viable vaccine has gone through laboratory and animal testing, it goes through a series of clinical human trials that will involve hundreds of participants and, when it comes to testing safety, ultimately thousands.
It is important to realise that in a disease like COVID-19 for which there are no therapies and can be fatal, no so-called ‘human challenge trials’ will be undertaken where vaccinated participants are deliberately infected with the pathogen to test the response.

Instead, the initial, or Phase 1 trial, will involve giving the vaccine to a randomly selected group of healthy individuals and then testing whether they produce the correct antibodies, in the required quantities, compared with those not given the vaccine.
This will establish whether the vaccine can generate the targeted immune response that is believed to be sufficient for protection.

Health & Medicine
Immunity to COVID-19: Lessons from malaria
A Phase 2 effectiveness trial will then test whether this response is consistent in more ‘real world’ settings across diverse populations – covering different ages, genders, medical backgrounds and pregnant women. They provide an early signal of common vaccine side effects.
Finally, in Phase 3, the vaccine is trialled in a larger population where the disease is present and participants are monitored for their results.
These trials are needed to prove that vaccine-induced immune responses are protective against infection or severe disease outcomes, and to detect any less frequent side effects.
The entire process involves ongoing safety checks before the vaccine can be approved.
There is an emerging assumption that we could have a COVID-19 vaccine within 12 to 18 months. It would seem implausible that a vaccine could come any earlier than that, given the urgency and the potential for simultaneous trials speeding up the process.
But, realistically, a vaccine could take years.

That means we may for a time have to adapt to life with COVID-19, maintaining good personal and environmental hygiene, adopting physical distancing practices and continuing with public health measures including case isolation, and quarantine for contacts and travellers.
Perhaps the closest equivalent in living memory to what that could be like may be the periodic community outbreaks of polio before a vaccine was developed in the 1950s.
Developing a vaccine that can do a better job against a disease than our own immune systems is always incredibly challenging and can’t be taken for granted.
Health & Medicine
Understanding the response of our immune system’s powerful army
The objective of any vaccination program is to ensure that a large enough proportion of a population are immune to an infection so that the infection itself effectively dies out because it can’t continue to spread.
Even people with no immunity will end up protected because the virus or bacteria is encountering too many immune people who block it from being passed on.
When this point is reached it is called “herd immunity” – everyone is effectively immune because enough of us are immune.
How big a proportion of a population needs to be immune to achieve herd immunity depends on how infectious a particular pathogen is.
The measles virus is highly contagious and requires at least 95 per cent of a population to be immune. This is why when people aren’t vaccinated against measles we still get pockets of outbreaks.
Herd immunity for seasonal influenza needs perhaps only a third of the population to be immune as each person on average only passes it on to 1.5 others (in a fully susceptible population).

Health & Medicine
The COVID-19 treatment trials that ‘learn as they go’
Initial studies suggested that COVID-19 had an infection reproductive rate (R₀) of rate of something over two, which means an infected person could be expected to infect at least two others in a fully susceptible population.
If that is the case, for an R₀ of 2.5 herd immunity would be achieved in theory at 60 per cent of the population being immune, either because they are immune from having been infected in the past or through having had a vaccine.
In practice, it may be more than 60 per cent due to the diversity in the population when it comes to contact patterns. There are also studies suggesting higher infection rates for COVID-19, which would mean that the threshold for herd immunity would also be higher.
But this assumes a vaccine is 100 per cent effective in everyone who has it, and this is rarely the case.
We know for instance that the effectiveness of the annual influenza vaccine usually varies from around 40 to 60 per cent.
It is hard to know how effective any COVID-19 vaccine is going to be, but it is probably wise to plan it will be potentially much less than 100 per cent.
That means if we are relying on a vaccine to control COVID-19, we will need much higher coverage than 60 per cent – or permanent changes to how we socialise and interact so that a mix of changed social behaviour and an okay (but not perfect) vaccine together can get us to herd immunity.
Once a vaccine is created, available supplies will take time to build, which means it won’t be available for all countries at once. It may be that it is prioritised for particularly exposed countries, like poorer nations with inadequate health systems.
Health & Medicine
The dynamics of disease
For example, the global vaccine alliance, GAVI, backed by the United Nations and the Gates Foundation, uses its buying power on behalf of poorer countries to make vaccines more affordable.
Australia is unlikely to be the first in line.
Once Australia does have supplies, we are fortunate in that we already have the distribution infrastructure in place. But it isn’t straight forward.
For example, vaccines need to be kept at cool temperatures during transportation and storage – between two and eight degrees Celsius.
We have this cold chain in place already, but we have not attempted to vaccinate a whole population for many decades. It will take planning and time.
Finally, decisions will have to be made on how we scale up the rollout – which groups of people should get it first?

Health workers are likely to be at the front of any queue (not only to because they are at higher risk of COVID-19 infection, but also because they risk passing it on to vulnerable people).
Beyond that group, the decisions may be more difficult.
For example, if the country is still practicing physical and social distancing when a vaccine becomes available, does it make sense to first vaccinate a lower risk group like the young so they can go about getting the economy moving faster? Or do you prioritise the elderly and vulnerable for the vaccine like we do with influenza?
The best course will depend on the circumstances we find ourselves in only once a vaccine becomes available.
Banner and graphics: Jared Henderson and Jonathan Liew/University of Melbourne
Journalist: Andrew Trounson/University of Melbourne