Health & Medicine
The Global Cancer Atlas

Cancer treatment is largely based on where it originates in the body, but when a primary site can’t be found, genomics is helping guide diagnosis and treatment for cancers of unknown origin
Published 9 June 2021
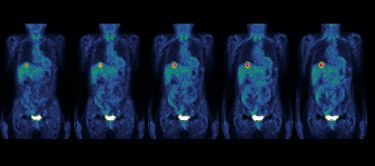
One of the first questions for any new cancer diagnosis is ‘where in the body did the cancer originate?’
In most cases, this can be answered through a series of clinical investigations, including cancer imaging and a pathological diagnosis from a tumour biopsy.

But even with the use of modern diagnostics, the origin of a patient’s cancer is not always apparent once it has spread (or metastasised), resulting in a diagnosis of ‘cancer of unknown primary’ or CUP.

Health & Medicine
The Global Cancer Atlas
According to recent Australian cancer statistics, approximately 2,400 Australians are diagnosed with CUP each year, ranking it 14th among all cancer diagnoses. But with only 13 per cent of patients surviving five years or more, CUP is the sixth most common cause of cancer-related deaths.
Because cancer medicine is still largely based on a cancer’s anatomical location, CUP can represent a significant challenge to standard treatment practices.
Even when treated with broad-acting chemotherapy, without a definitive cancer diagnosis these treatments may, at best, only extend a CUP patient’s life by a few months.
Providing hope for a range of patients, including those with CUP, precision medicines are helping researchers and clinicians target cancers once thought to be incurable.
These therapies rely upon knowledge of a cancer’s “Achilles heel”, rooted in the gene changes or mutations that enable the cancer to grow, spread and evade the body’s intrinsic anti-cancer defences.

“I remember sitting with Mum during her first oncologist appointment,” says Kym Sheehan. Her mum was diagnosed with CUP in 2013, and there were few resources to help them understand what a CUP diagnosis meant.
Kym has since been working with our research team in a patient advocacy role.

Health & Medicine
Personalising bowel cancer detection
“The doctor was weighing up the conflicting evidence from the cancer markers in Mum’s blood samples.
“But if we had another way for medical professionals to make a diagnosis, CUP patients like my mum could receive more effective treatment, including the potential for access to novel treatments,” she says.
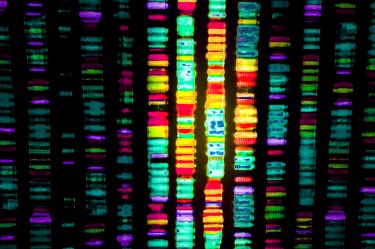
Over the last 20 years, our team and many others have contributed to huge advances in our understanding of the gene mutations linked to different cancer types by analysing the cancer genomes – the complete set of genes in a tumour – from hundreds of patient samples.
This information has provided detailed genetic portraits of both common and rare diseases, enabling access to a growing number of precision treatments that act by specifically inhibiting defective genes.
This is especially good news for CUP patients because knowledge of the genetic drivers of disease becomes as important, if not more important, as knowing a cancer’s origin.
We are now embarking on a study to confirm that state-of-the-art genomics can help to extend a patient’s life span, as well as improve a patient’s experience and reduce the overall healthcare costs associated with a CUP diagnosis.

To date, our work through the national Solving Unknown Primary Cancer study (or SUPER) has focused on the use of targeted gene panels, which allows us to study up to a few hundred genes at a time.
Health & Medicine
The genomic clues to disease
The study has helped diagnose the tissue of origin of the cancer and identify cancer-specific genetic defects in more than half of the 200 cases recruited.
This allows clinicians to confirm current cancer management plans or inform a change in treatment and more targeted therapy and clinical trials – with many treated through clinical lead Professor Linda Mileshkin’s specialised CUP clinic at the Peter MacCallum Cancer Centre.
More recently, we have shifted our attention to using whole-genome sequencing, enabling the analysis of all tumour genes. This is done through DNA sequencing platforms established by Professor Sean Grimmond and Associate Professor Oliver Hofmann at the University of Melbourne Centre for Cancer Research.
Surveying the entire tumour genome allows us to detect mutational patterns, beyond those found in panel data, including valuable diagnostic information on the life history of the cancer and its potential therapeutic vulnerabilities.
This is the focus of the SUPER-NEXT project – to understand the clinical impact of whole-genome sequencing, for an additional 200 CUP patients from 16 sites across Australia.
In addition to genomic testing of DNA taken directly from a patient’s tumour, the project will also pilot the potential of non-invasive blood tests – or liquid biopsies – for the detection of tumour DNA circulating in the blood, offering an easier and potentially quicker method to interrogate mutated genes in a patient’s tumour.

To provide a holistic approach to improve the outcomes of CUP patients, our unique multidisciplinary team of experts in cancer genomics, medical oncology, cancer imaging, pathology and health economics also incorporates the psychosocial needs of CUP patients.
Preliminary results from research led by behavioural scientist and co-lead of the SUPER team, Peter Mac and Swinburne University’s Professor Penelope Schofield, show that CUP patients suffer disproportionally from their disease.
This is due to the uncertainty that is inherent with such a diagnosis, the inequity in access to treatments and lack of patient resources and support mechanisms.
Also providing important insights into the unique psychosocial and experiential challenges associated with a CUP diagnosis are members of a dedicated SUPER consumer group.
Kym, who is a core member of this group, believes that removing as much uncertainty from the initial diagnosis of CUP is a priority.
“We rely on our knowledge of cancer from daily life; usually people are diagnosed with a cancer of ‘something’ like breast, lung or prostate cancer – but a CUP diagnosis does not fit this experience,” she says.

“As CUP always involves metastatic cancer, patients and their carers need targeted information to guide the many decisions they need to make.”
“That includes understanding what a genome sequencing test involves, what the outcome of this test means, what are the treatment options and, most importantly, what quality of life can the patient reasonably expect.”
A major goal of the SUPER-NEXT study is to help patients and their carers understand not only their disease but also how new innovations in science and technology may help improve their diagnosis and treatment.
The potential health and societal impacts of this work are significant.
By increasing the duration and quality of life for CUP patients, and decreasing the cost and burden on the healthcare system, this study has the potential to benefit not only people affected by CUP, but the broader community as a whole.
SUPER-NEXT is a collaborative study co-led by research and clinical partners at the University of Melbourne and Peter MacCallum Cancer Centre. The study is funded by the Medical Research Future Fund and is also supported by the University’s industry partner Illumina, a leading international genomics company.
SUPER-NEXT builds on a pilot study supported by the Australian Genomics Health Alliance using whole-genome sequencing to identify additional diagnostic features that may be helpful in a patient’s diagnosis or direct treatment. It also informed the technical limits of whole-genome sequencing and testing of new bioinformatic methods for predicting cancer tissue of origin.
Banner: Getty Images