Health & Medicine
What it takes to make a heartbeat
Cardiovascular disease is a global killer. Now researchers have developed a fast, inexpensive and scalable method for engineering blood vessels from natural tissue
Published 1 August 2023
Blood vessels keep us alive. They are the highways that transport oxygen-rich blood and nutrients to all corners of our body – feeding our tissue and organs – while simultaneously removing toxic waste products.
Blood vessel disease and dysfunction can result in life-threatening situations like heart attack, stroke and aneurysm. Blood vessel failures are a major reason why cardiovascular disease is the number one killer globally.

Bypass surgery is often used to replace severely diseased blood vessels. Non-living grafts made of synthetic polymers can be used in many cases.
However, small diameter blood vessels – like the coronary artery that feeds blood to the heart – cannot be replaced with synthetic vessels because blood will clot on their surface and obstruct the graft.
In these cases, a less essential blood vessel is taken from elsewhere in the body and used to re-route blood around the diseased vessel, restoring blood flow to the starving tissue.
If successful, bypass surgery can add many more healthy years to a patient’s life.

Health & Medicine
What it takes to make a heartbeat
Bypass surgery is a lifesaving treatment, but there are significant limitations. Most pressingly, some patients lack appropriate donor vessels due to previous surgeries or comorbidities like diabetes which means treatment options for these patients are limited.
But what if we could instead manufacture ‘real’ blood vessels to treat these patients?
Tissue engineered blood vessels – blood vessels that are fabricated using human cells and tissue – could provide a viable treatment option.
Additionally, we could use these vessels for many other purposes – like creating a built-in blood supply when engineering larger tissue constructs. This isn’t currently possible because the tissue would die when implanted into the body.
Despite the need for tissue engineered blood vessels, successfully creating them has proved challenging. Blood vessels are complex, multilayered tissues and their structure is intimately tied to their performance.
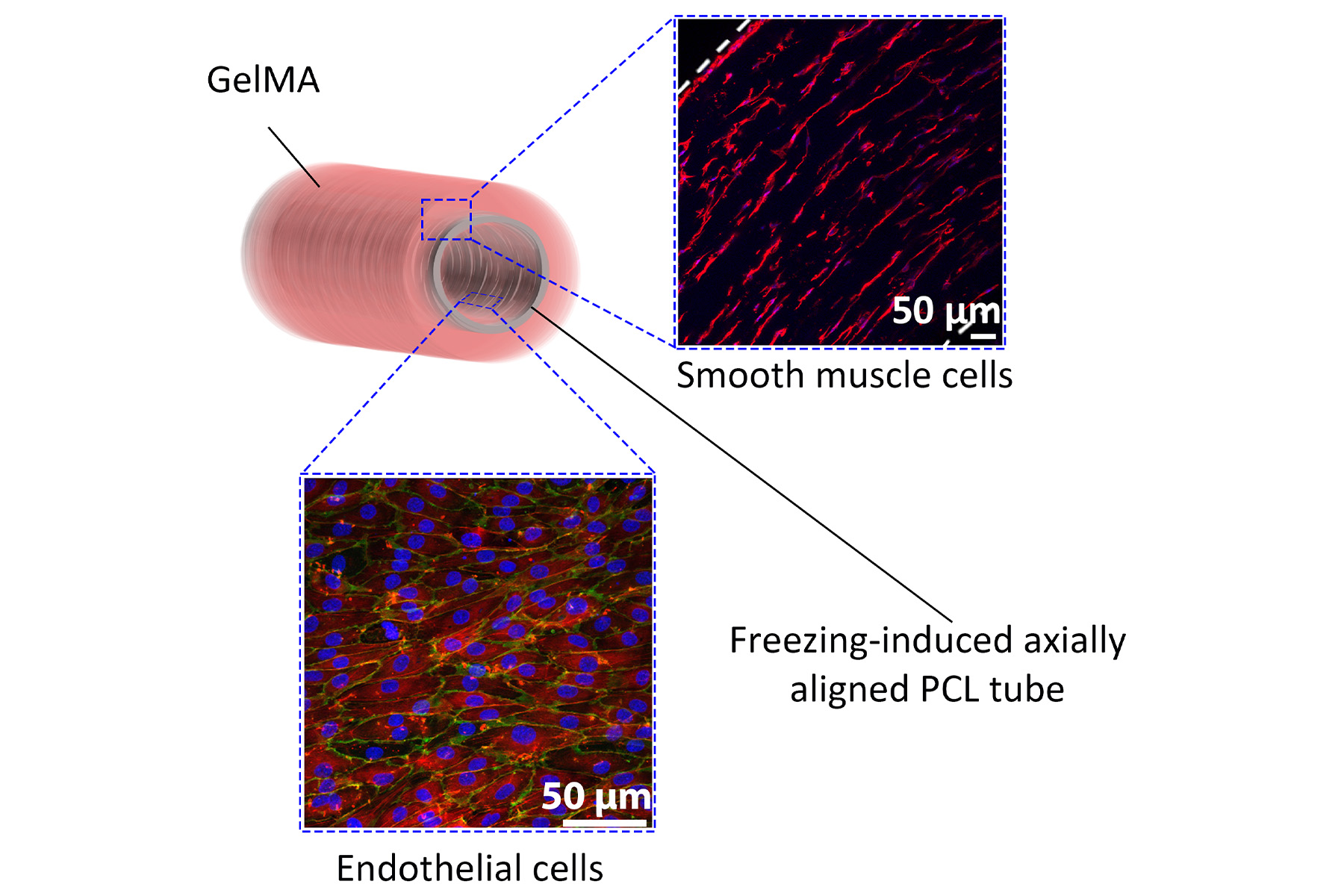
The inner-most layer of a blood vessel is the endothelium layer – this is a single layer of specialised cells that align along the axis of the blood vessel, supporting blood flow and preventing coagulation.
Surrounding the endothelium is a 3D layer of smooth muscle cells that wrap like a series of rings around the blood vessel. This provides the blood vessel with mechanical strength to prevent rupture – contracting and relaxing to regulate blood pressure.
Health & Medicine
Get your heart checked
Researchers around the world have been trying to perfect blood vessel tissue engineering for many years.
However, current methods are slow, require specialised and expensive equipment (like bioreactors), and are low throughput – meaning it’s difficult to provide the needed supply of engineered vessels.
By combining multiple materials and fabrication technologies, our team have developed a fast, inexpensive and scalable method for tissue engineering blood vessels.
And, as we report in the journal ACS Applied Materials and Interfaces, our vessels replicate the complex geometry of native blood vessels.
They are not quite ready for bypass surgery, but we’re hopeful that we’re on the right track.
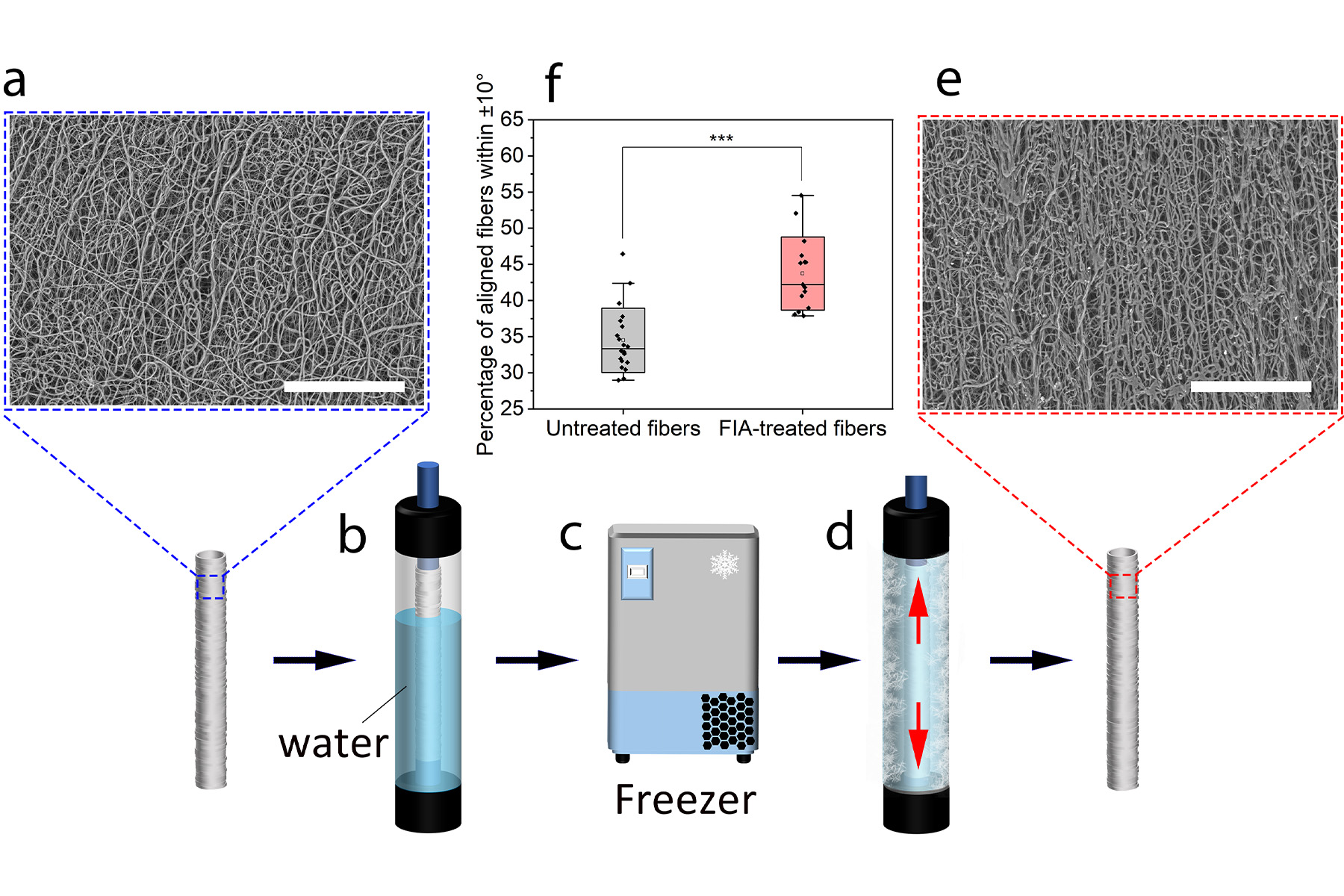
First we needed to create the shape, a kind of framework on which to grow the blood vessel layers. We did this by electrospinning a layer of polymer fibres onto a mandrel, which provides the tubular shape for the blood vessel graft.
Electrospinning is a technique that uses an electrical voltage to draw a polymer stream into thin fibres that mimic the protein structure of our native tissue, a bit like spinning wool onto a bobbin at the nano-scale.
However, this process results in fibres that are randomly oriented, when we need fibres aligned along the length, or axis, of the tube to promote axial alignment of the endothelial cells.
To align these fibres, we developed a simple freezing technique.
By placing the electrospun tube into a rigid mold partially filled with water and freezing it, we caused ice crystals to grow along the axis, which pushed the fibres into alignment.

Sciences & Technology
Harvesting big energy from small movement
We then grew endothelial cells on the tube to create the inner layer of the vessel – the endothelium. The cells spontaneously align with the fibres, generating a continuous, aligned endothelial cell layer like we see in native blood vessels.
This layer also provides appropriate mechanical properties, enables the graft to be sutured to native blood vessels and prevents rupture of the graft.
Next, we cast a soft hydrogel layer around the electrospun fibres. This hydrogel layer prevents leakage from our graft and also acts as a scaffold for smooth muscle cells.
We know that cells are very sensitive to the stiffness of their surroundings so we trialled hydrogels of varying stiffness.
Surprisingly, we observed that the softer gels allowed the vascular smooth muscle cells to rapidly and spontaneously align in a 3D ring structure, mimicking what is found in native blood vessels.
We are now able to rapidly and cheaply manufacture blood vessels using living tissue that have appropriate mechanical properties and mimic the cellular orientation of endothelial and vascular smooth muscle cells in native blood vessels.
This research advances our ability to engineer human blood vessels but work still needs to be done before these blood vessels can progress to the clinic.
Our team has designed the electrospun polymer layer to degrade over time, yielding a fully biological blood vessel. So, we need to verify that the electrospun layer degrades at an appropriate rate, or else the graft could lose integrity and rupture.
In the future we hope these engineered blood vessels will be used to treat cardiovascular disease – especially in those vulnerable patients who lack appropriate donor vessels.
Banner: Getty Images