Health & Medicine
Why the number of dementia cases has doubled

Plant biologists have described how a protein called CC1 helps plants to grow under salt stress and, intriguingly, it shares features with a protein linked to Alzheimer’s disease
Published 20 February 2019
Alzheimer’s disease and other forms of dementia are a growing burden on society and a leading cause of death among the elderly. There is no cure.
Salt from rising water tables and sea water intrusions is increasingly affecting crop production around the world. It’s estimated that up to 50 per cent of irrigated land worldwide could be impacted by salination.
Non sequitur? Not necessarily.

New research led by the University of Melbourne and FMP Berlin has found a fascinating link between these two global issues that is opening new areas of research in both plant biology and neuroscience.
The common thread appears in certain proteins associated with microscopic structures called microtubules; cylindrical filaments that play a key role in guiding the transport of material in cells.
In plants, microtubules guide the synthesis of cellulose, a major cell wall component that controls cell shape and is the basis for wood formation. In the brain, microtubules help transport materials inside neurons, the building blocks of the brain and nervous system.

Health & Medicine
Why the number of dementia cases has doubled
Microtubules use associated proteins to transport material, while other proteins help to keep them intact.
A protein supporting microtubule function is Tau, which was discovered at Princeton University in 1975. Dysfunctional Tau has been implicated in neurodegeneration, which can ultimately lead to Alzheimer’s or a suite of other neurological diseases, and, eventually death.
For more than 20 years this protein has been seen by many as the key to an effective vaccine or other treatment for Alzheimer’s.
CC1, a plant microtubule binding protein, has a more recent history.
In 2015, Professor Staffan Persson, currently an Australian Research Council Future Fellow at the University of Melbourne, led a study by Dr Anne Endler and Dr Christopher Kesten looking at how plants cope with salt stress.

“We found the proteins we call CC1 and CC2 were in some way able to protect a plant’s ability to produce cellulose – a prerequisite for the plant to grow – during exposure to salt stress,” Professor Perrson says.
Dr Kesten, co-lead author of the new study published in Nature Communications and now a researcher at ETH Zurich, says the CC1 protein works on multiple stages of the cellulose synthesis process.
“We know that part of the CC1 protein binds to the cellulose synthase proteins in the cell membrane, but the protein also contains a part on the inside of the cell that binds to microtubules,” he says.

Health & Medicine
How much exercise keeps our brains healthy as we age?
“And that seems to be what is really important for the proteins to help the plant to withstand salt stress. So, we were really interested in the part of the protein that binds to microtubules.”
Professor Persson, Dr Kesten and their colleagues in Australia, Germany and Switzerland then set out to better understand the structure of CC1 and how it worked, and in the process found an intriguing link between CC1 and Tau.
“We took a multitude of different approaches to try and decipher how CC1 binds to microtubules, locate the binding regions in the proteins, and assess if we could mutate some of those to abolish the microtubule-related function of the proteins,” says Dr Kesten.
Mr Arndt Wallmann, a PhD student at FMP Berlin and co-lead author of the study, says the team got excited when they found that CC1 has four linear motifs that attach directly to microtubules.
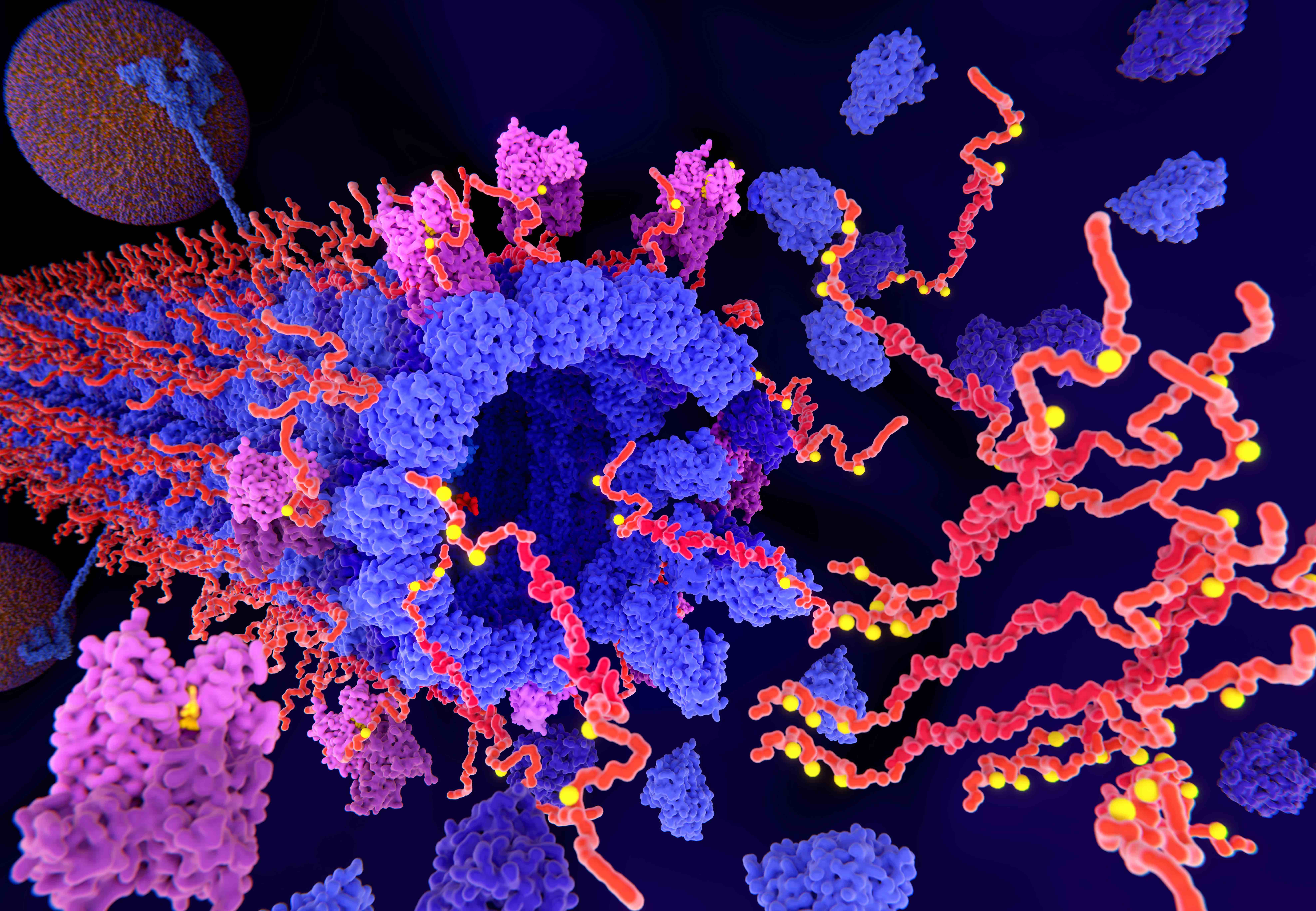
“The binding motifs are spaced in a certain way, and when we looked at that spacing between those four domains and the highly dynamic properties of the binding, it’s very, very similar to what you see in the Tau protein,” says Mr Wallmann.
“That got us pretty excited because Tau is probably the most studied microtubule protein in biology – if you look up Tau on MEDLINE you will find thousands of papers.”
“People have looked for quite some time for close relatives of Tau in other kingdoms but none have been found in plants,” says Professor Persson.
Health & Medicine
‘Rusty’ brains linked to Alzheimer’s
Professor Oschkinat, from FMP Berlin, explains that the similarities don’t end with the number and spacing of binding domains, making the connection between the two even more interesting.
“Nuclear Magnetic Resonance showed us that CC1 is an unstructured protein, in that it doesn’t have the typical sheet-like or helical structure found in most proteins. That’s also the case for Tau,” he says.
“Hydrophobic amino acids are enriched in the microtubule binding motifs of CC1. That’s also found in Tau. And when we matched the segments of amino acids that make up the microtubule binding domains of the two proteins, they are surprisingly similar in composition.”
The obvious conclusion from this would be that Tau and CC1 both come from a common, ancestral protein that existed before plants and animals took different branches in the tree of life, but the lead authors don’t think this is the case.
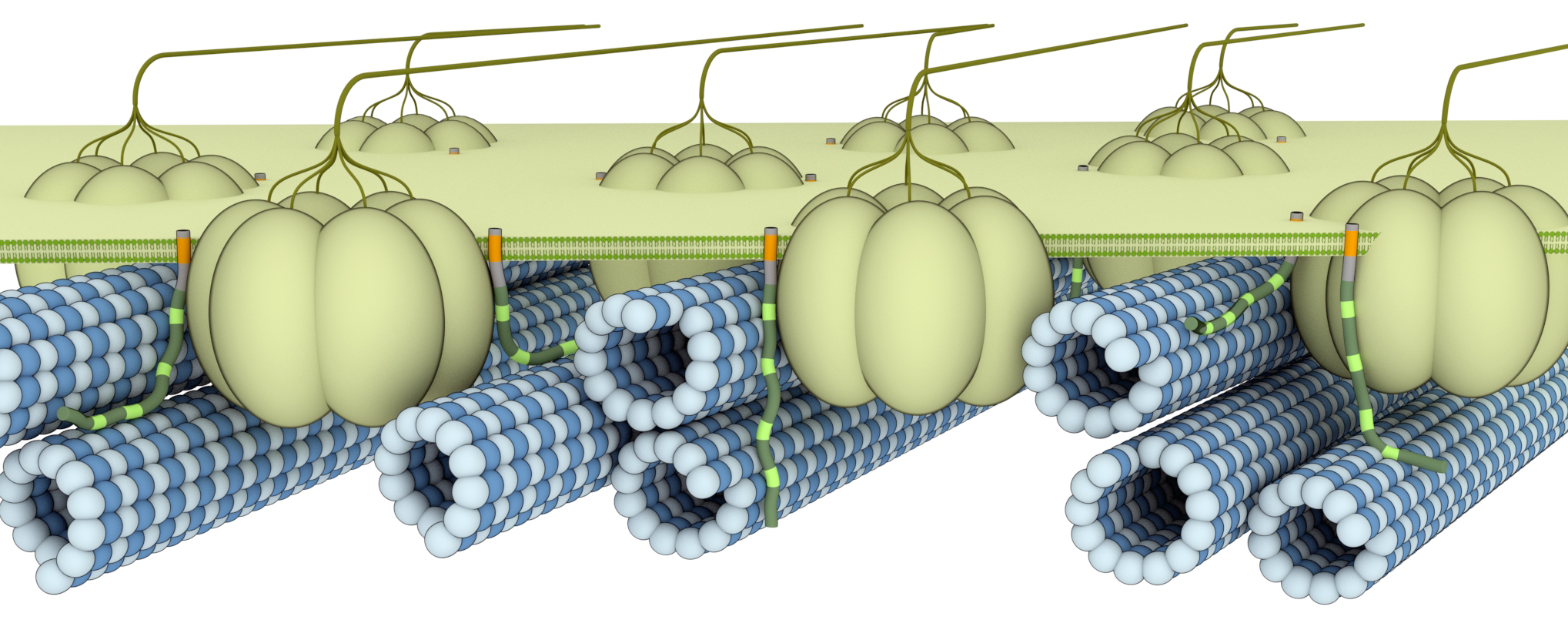
“One possibility is that this shared function might have come about via convergent evolution, so basically you start with two, relatively unrelated proteins, and then the function of those proteins converges over time into being similar, yet they probably come from different ancestor proteins,” says Professor Persson.
“The reason we think this, is that Tau has other protein domains as well, and we can’t find those in the CC1. Likewise, CC1 is a trans-membrane protein, so it spans the cell membrane, and that’s not the case for Tau,” Dr Kesten says.
“So apart from these microtubule binding or bundling or stabilising functions, the other parts of the two proteins likely have completely different functions,” Mr Wallmann adds.

Environment
Unlocking the inner workings of plant growth
Having found the link, the researchers are now trying to combine the two proteins, CC1 and Tau, to better understand how they work.
“We’ve screened the first batch of seeds where we took the CC1 and exchanged the piece that is inside the cell for the correlated part of Tau,” says Professor Persson.
Professor Persson thinks that by doing this type of manipulation they can change the function of the microtubule binding capacity of the proteins.
“Adding part of Tau into CC1 might not be ideal for the function of this protein in the plant cell, but it might teach us quite a lot about how this interaction is happening and how this affects the protein function,” he says.
“And that could also be very important for our understanding of the protein in neurons.”
Banner: Barth-Jan van Rossum/FMP Berlin