How a mathematical equation opened a new frontier in nanotechnology
Frequencies reveal the shape and weight of nano-scale objects
Published 31 August 2015
A University of Melbourne mathematician has opened up a new frontier in mass spectrometry with a simple mathematical trick that for the first time simultaneously reveals both the shape and weight of tiny objects like viruses, bacteria and proteins.
Professor John Sader gained mathematical immortality in the 1990s when a method – really a series of equations – he developed to calibrate the miniature tips of atomic force microscopes was named after him.
Continuing his research into the mathematics of the very small, he has now crafted an elegant mathematical algorithm that allows scientists to tell both the shape and weight of a single molecule, using devices one-thousandth the width of a single human hair.

For a century, scientists have been measuring the weight of very small particles using a technique called mass spectrometry (MS). They add electrical charges to a group of particles and then accelerate them using an electromagnetic field. By measuring the rate of acceleration, they can determine the weight of the particles.
Prof. Sader explains that traditional mass spectrometry is like pushing a car.
If you push a light car, it will shift relatively easily, but if you try to push a bus with the same force it’s harder to move. So they apply a force, accelerate the molecule and measure how long it takes to hit a target. Newton’s Law tells us the heavier ones take longer than the light ones to get there.
Mass spectrometry is brilliant at differentiating between particles of different weight, such as between carbon and nitrogen, or even the different isotopes of nitrogen, or between a large and a small protein or virus particle. But this method can’t distinguish between totally different particles if they have the same weight.
For example, using only the weight of an object as a measurement might trick scientists into thinking something is an orange when it’s actually a banana. The molecules can weigh the same, but have shapes that are completely different. It has been impossible to tell them apart using this technique – until now.

The first breakthrough came when a team at the California University of Technology, led by Professor Michael Roukes, developed completely new way to do mass spectrometry. They built a system that uses tiny vibrating devices, called Nanoelectromechanical systems (NEMS), to precisely weigh single molecules, like proteins, one at a time.
When a particle or molecule lands on a NEMS, the additional weight changes the frequencies at which the device vibrates.
If traditional mass spectrometry is like pushing a car, Prof Sader says NEMS mass spectrometry (NEMS-MS) is more like plucking a guitar string. When plucked, a guitar string doesn’t just vibrate at one frequency; it has many frequencies that combine to give the string its pitch and tone. If on object, like a drop of solder, attaches to the string, the pitch and tone of the string change because the frequencies of the string change.

The same thing happens when a molecule attaches to the NEMS. The changes in the frequencies of the NEMS – the frequency shift signature – can be used to determine the molecule’s weight.
The second breakthrough came in 2012-2013 when Prof. Sader was invited to Caltech as the Kavli Nanoscience Institute Distinguished Visiting Professor in Physics. While there, he started thinking about NEMS-MS and whether there was a better way of calculating the weight of a particle, and what other information the frequency shift signature could give about the particle.
He soon came up with a way to drastically simplify the mathematics required to calculate the weight of an object using NEMS-MS.
Prof. Sader then realised that the frequency shift signature gave not only a unique ‘fingerprint’ for the weight of the object, it also contained information about how the weight was distributed in three-dimensions – in other words its shape.
Suddenly bananas and oranges began to look very different.
The set of mathematical equations that Prof. Sader developed, called the ‘inertial imaging’ algorithm, has revolutionised the field of mass spectrometry. This research was recently published in Nature Nanotechnology.
“The new approach uses state-of-the art nanodevices and frequency measurements. The inertial imaging algorithm reinterprets those measurements to get new information about the molecule’s shape,” Prof. Sader says.
If you can start to identify shapes, then you can build up a database of what single molecules on a surface look like. Then you can start building up libraries and try to understand why molecules behave the way they do.
Prof. Sader and his collaborators are working hard to realise this new technology. He says that great ideas can be discovered in an instant, but technological revolutions take longer.
“We’ve proposed the technique, we’ve done the engineering design, demonstrated proof-of-principle measurements, and it tells us we can go all the way down to the atomic level,” he said.
“This gives us the blueprint to now make a working nanodevice that can simultaneously weigh and image single molecules, with atomic-scale resolution.”
So far, the new technique has been validated using particles scientists already know the shape and size of, such as polymer nanodroplets.
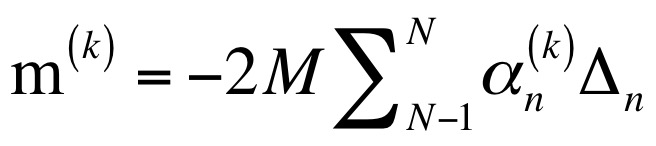
Prof. Sadar says refining the technology to a point where it can achieve atomic scale resolution to categorise objects with unknown shapes will take time, but he’s confident it will happen.
Professor Michael Roukes, who leads the team at Caltech who are refining this technology, describes it as putting together a composite sketch of a criminal. Every piece of information you can gather helps. He explains that Prof Sader’s discovery will markedly speed up the discovery process.
“You can imagine situations where you don’t know exactly what you are looking for, where you are trying to figure out the body’s immune response to a particular pathogen, for example,” Prof Roukes said.
“A suspect is much more likely to be identified from a composite sketch that accurately depicts their brown crew-cut hair, blue eyes, aquiline nose, and goatee beard, than from one showing a largely featureless blob with a smattering of facial hair.
If you are looking to identify someone, every separate bit of information that we can add to the picture is important, especially when you are trying to identify only one individual from a crowd.
“We say that cancer begins often with a single cell, so there is something different about the molecular composition of that one cell. With this technique, we potentially have a new tool to figure out what is unique.”
In the nano-world, sometimes you need to play a guitar to tell an orange from a banana.
