
Sciences & Technology
The Quantum sensing revolution

Scientists have used new quantum magnetic imaging techniques to study the hardest known biomineral – the magnetite found in mollusc teeth – which may inspire new industrial materials
Published 10 March 2020
Biology provides us with a constant source of inspiration for designing and exploring new functional materials.
Velcro for example arose from how plant burrs hook onto clothes, and the nose of bullet trains follows the design of a Kingfisher’s beak. The science of adapting natures’ designs to solve complex engineering challenges is known as biomimicry.

Now, our research team from the University of Melbourne, the University of Western Australia and the University of California, Riverside, have turned to a common sea mollusc, the chiton, for clues on how to engineer lightweight, hard and abrasion-resistant materials in a clean and energy efficient way.
The chiton Acanthopleura hirtosa, found in the inter tidal zones of Australia’s coastline, mineralises its own teeth using iron extracted from sea water to create a magnetite tooth coating. This substance is the hardest known biomineral, tougher than stainless steel.

Sciences & Technology
The Quantum sensing revolution
The chiton’s magnetic teeth resemble iron-covered spoons assembled in a conveyor belt-like organ, known as the radula. New teeth are constantly produced to replace those worn away while feeding on algae found within the rocks on which they graze.
We hope to learn and adapt the design principles of mineral layering in the chiton teeth to deliver low-cost, energy efficient functional materials that can be applied across industrial applications including surface coatings in construction, mining
and medical applications, contrast agents for medical imaging and water purification.
Magnetite is currently produced using energy intensive techniques using high temperatures and strong acid and base chemicals. In contrast, chiton have evolved and optimised this process to assemble superior materials in sea water at 15-20 °C, by extracting iron from their sea water surroundings.
One of the most difficult aspects of biomimicry is understanding the fundamental building blocks and mineral growth processes that are used in nature.

By applying new magnetic microscopy techniques, pioneered at the University of Melbourne, our team was able to study how these animals begin to assemble these unique materials at the nanoscale.
The imaging technique uses a thin sheet of synthetic diamond crystal about four millimetres square. To create the sensors we remove two carbon atoms from the usual diamond structure, replacing them with one nitrogen atom and leaving an atomic space, or vacancy, where the other carbon atom should be.
Sciences & Technology
Quantum 2.0: At the beating heart of biology
The combination of the nitrogen atom, the vacancy and an additional electron creates the so-called Nitrogen Vacancy (NV) defect, which acts as the sensor.
When green light from an optical microscope is shone onto the diamond surface the NV defects reflect back red light, the strength of which is dependent on the local magnetic field.
The NV defects are incredibly sensitive and can detect magnetic fields one million times weaker that your standard fridge magnet.
This sensitivity allows us to pinpoint the source of the magnetic field from the iron biominerals, and correlate its position within the tooth.
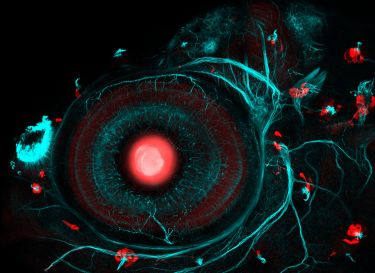
Using the diamond magnetic microscope, we have now produced the first magnetic image of chiton teeth in the early stages of mineralisation. The magnetic field was imaged from magnetite nanoparticles as well as its precursor iron biomineral, ferrihydrite.
The maps allow us to visualise the mineralisation pattern used by the chiton to convert ferrihydrite into magnetite in the developing teeth, with imaging resolution one hundred times smaller than the width of a human hair.
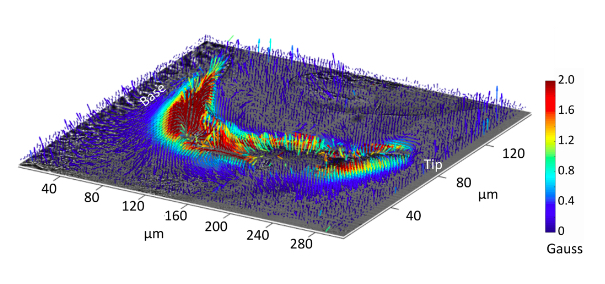
What we see is that the teeth recruit ferrihydrite from both the anterior and posterior sides of the teeth (from the front and back surfaces of the tooth) to drive the magnetite mineralisation.
More interestingly, when we look at the magnetic field from the magnetite nanoparticles, we find that the magnetite magnetic domains are aligned and ordered across the entire tooth section.
Sciences & Technology
What has Quantum ever done for me?
This was an unexpected and fascinating finding as previous research using electron microscopy appeared to show no crystallographic ordering in these materials, however our magnetic images show that the individual magnetite nanoparticles that emerge in the early stages of mineralisation display a high degree of magnetic order.
This raises the question, is magnetism involved in the self-assembly of these ultra-hard materials?
To help answer this, our team will focus on applying the magnetic microscopy technology to image synthetic analogues in the hope of understanding how the magnetic properties impact the self-assembly of magnetite. We hope that this new knowledge can lead to the production of new bio-inspired magnetic materials with enhanced properties.
Learning from Nature is challenging, but new technology is helping unlock its secrets. Our research is yet another example of how quantum technology can be used to explore the complex world of biology.
Banner: Acanthopleura hirtosa chiton radula. The full radula consists of 73 teeth with various stages of mineralisation. The image above shows the teeth before and after the onset of magnetite mineralisation. The yellow/orange colour arises from the phase transformation from one form of iron (ferrihydrite) to another (magnetite). Image courtesy of Jeremy Shaw, University of Western Australia.